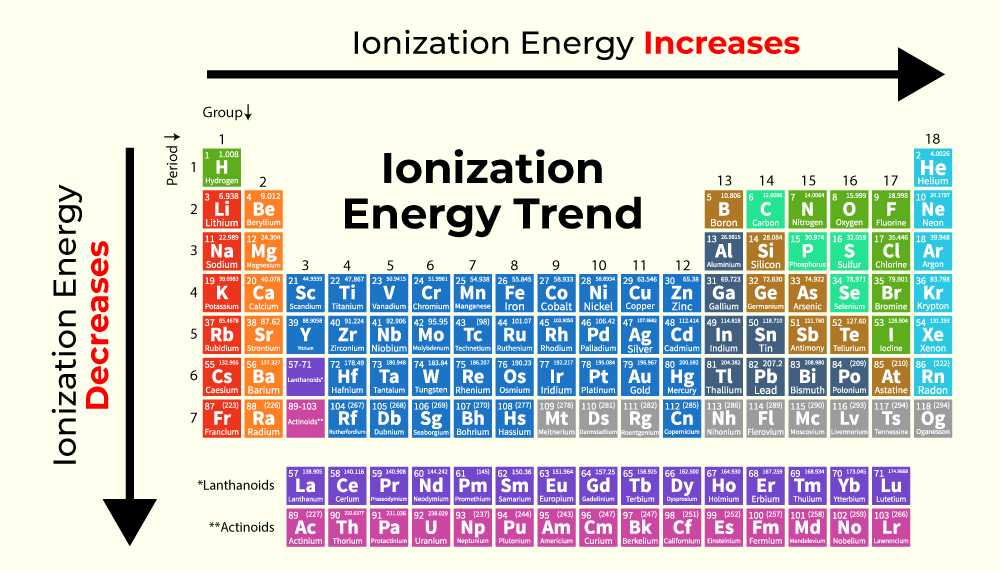
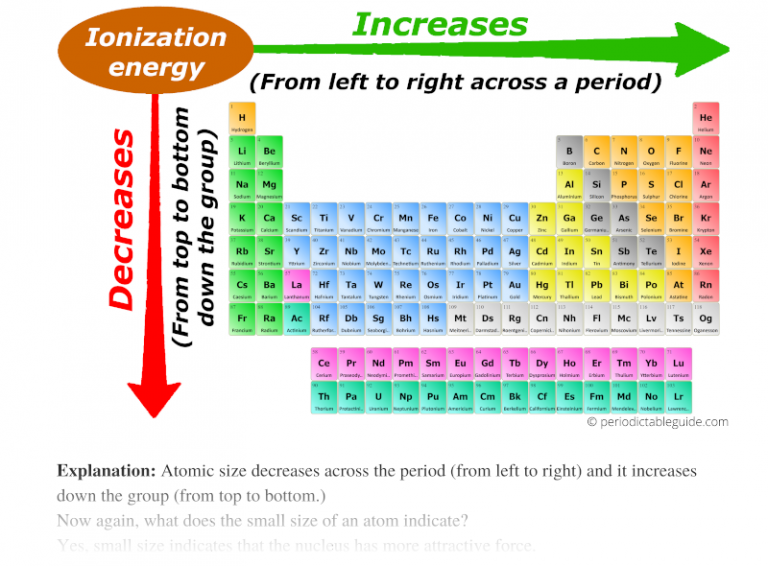
What Is The Group Period Trend Of Ionization Energy Dec 19 2015 nbsp 0183 32 Ionization Energy Trend in the Periodic Table General periodic trends In a group while moving from top to bottom it decreases It increases from left to right across a period 1 Trends in ionization enthalpy in a group The
Jan 1 2011 nbsp 0183 32 Use our revision notes to describe ionisation energy trends for A Level chemistry Explain trends in I E across periods and down groups of the Periodic Table Aug 28 2024 nbsp 0183 32 There are ionisation energy trends within periods and groups The ionisation energy increases as you remove more electrons from an element It is easy to remove electrons from a full subshell as they undergo spin pair repulsion
What Is The Group Period Trend Of Ionization Energy

What Is The Group Period Trend Of Ionization Energy
https://sciencenotes.org/wp-content/uploads/2020/07/periodicity-chemistry-1024x683.jpg

Compound Interest Periodicity Trends In The Periodic Table
https://i0.wp.com/www.compoundchem.com/wp-content/uploads/2014/02/Periodicity-Periodic-Table-Trends.png?ssl=1

Energy Level In Periodic Table
https://useruploads.socratic.org/H7ptw5aRSadPRg7SOPwa_ptable orbitals ionization energy.png
The ionization energy periodic trend is defined as a specific pattern in ionization energy displayed by the elements due to a change in its atomic structure The trend is observed across a period and down a group in the periodic table 4 8 Oct 27 2024 nbsp 0183 32 Revision notes on Ionisation Energy Trends amp Evidence for the AQA A Level Chemistry syllabus written by the Chemistry experts at Save My Exams
Sep 16 2020 nbsp 0183 32 Ionization energy increases moving across a period and decreases moving down a group There are exceptions to this periodic table trend Francium an alkali metal has the lowest ionization energy while helium Across a period from left to right the ionisation energy increases This is due to the increase in nuclear charge having a greater pull on the electrons and therefore more energy is required to
More picture related to What Is The Group Period Trend Of Ionization Energy
Ionization Energy Definition
https://media.geeksforgeeks.org/wp-content/uploads/20230310125901/ionization-energy-trend.png
Periodic Behavior Presentation Chemistry
http://www.sliderbase.com/images/referats/150b/(8).PNG
Periodic Trends Presentation Chemistry
http://www.sliderbase.com/images/referats/152b/(5).PNG
Ionization energy generally decreases from top to bottom in a given group that is column The latter trend results from the outer electron shell being progressively farther from the nucleus The general trend is for ionisation energies to increase across a period In the whole of period 2 the outer electrons are in 2 level orbitals 2s or 2p These are all the same sort of distances
General trends in the ionization energy of elements in the Periodic Table Ionisation energy decreases down a group Ionisation energy increases across a period from left to right May 27 2023 nbsp 0183 32 Ionization Energy Trend Across the period from left to right Increases Down the group from top to bottom Decreases The ionization energy of elements increases as we

Electronegativity Definition Value Chart And Trend In Periodic Table
https://www.chemistrylearner.com/wp-content/uploads/2016/06/Electronegativity-trend-periodic-table.jpg
Easy Defiintions For Words Associated With The Periodic Table Bowers
https://periodictableguide.com/wp-content/uploads/2020/12/ionization-energy-trend-in-periodic-table-768x566.png
What Is The Group Period Trend Of Ionization Energy - Across a period from left to right the ionisation energy increases This is due to the increase in nuclear charge having a greater pull on the electrons and therefore more energy is required to
.PNG)
.PNG)