What Is The Empirical Formula Of The Compound With The Molecular Formula C18h36 Convert the mass of each element to moles using the atomic masses from the periodic table Divide the moles of each element by the smallest number of moles calculated Round to the nearest whole number to find the atom ratio which represents the empirical formula
You can work out the molecular formula from the empirical formula if you know the relative mass formula M r of the compound Our expert help has broken down your problem into an easy to learn solution you can count on See Answer Question What is the empirical formula of the compound with the molecular formula C18H36 CH3 CH2 C9H20 C3H8
What Is The Empirical Formula Of The Compound With The Molecular Formula C18h36

What Is The Empirical Formula Of The Compound With The Molecular Formula C18h36
https://i.ytimg.com/vi/QlKvlgvKk8U/maxresdefault.jpg
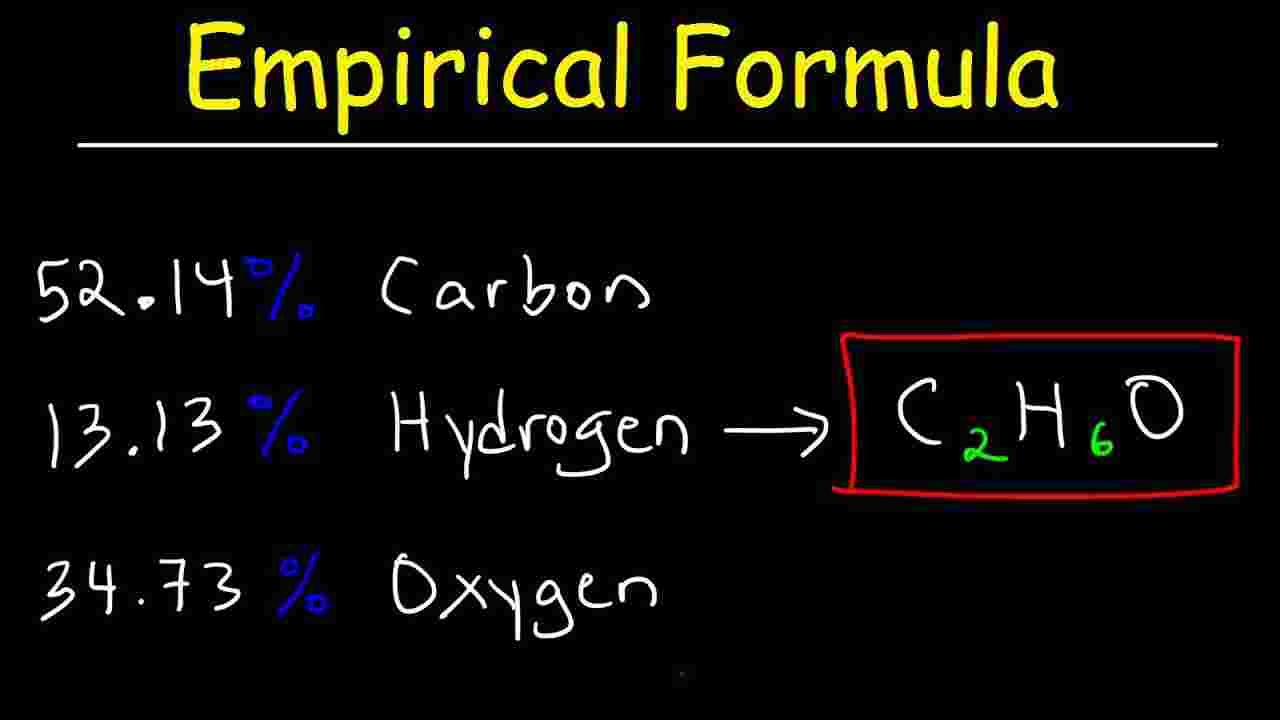
Emperical Formula Explanation And Worked Examples On Emperical Formula
https://www.len.com.ng/upload_blog_attachment/15e342249f09383.97127923.jpg
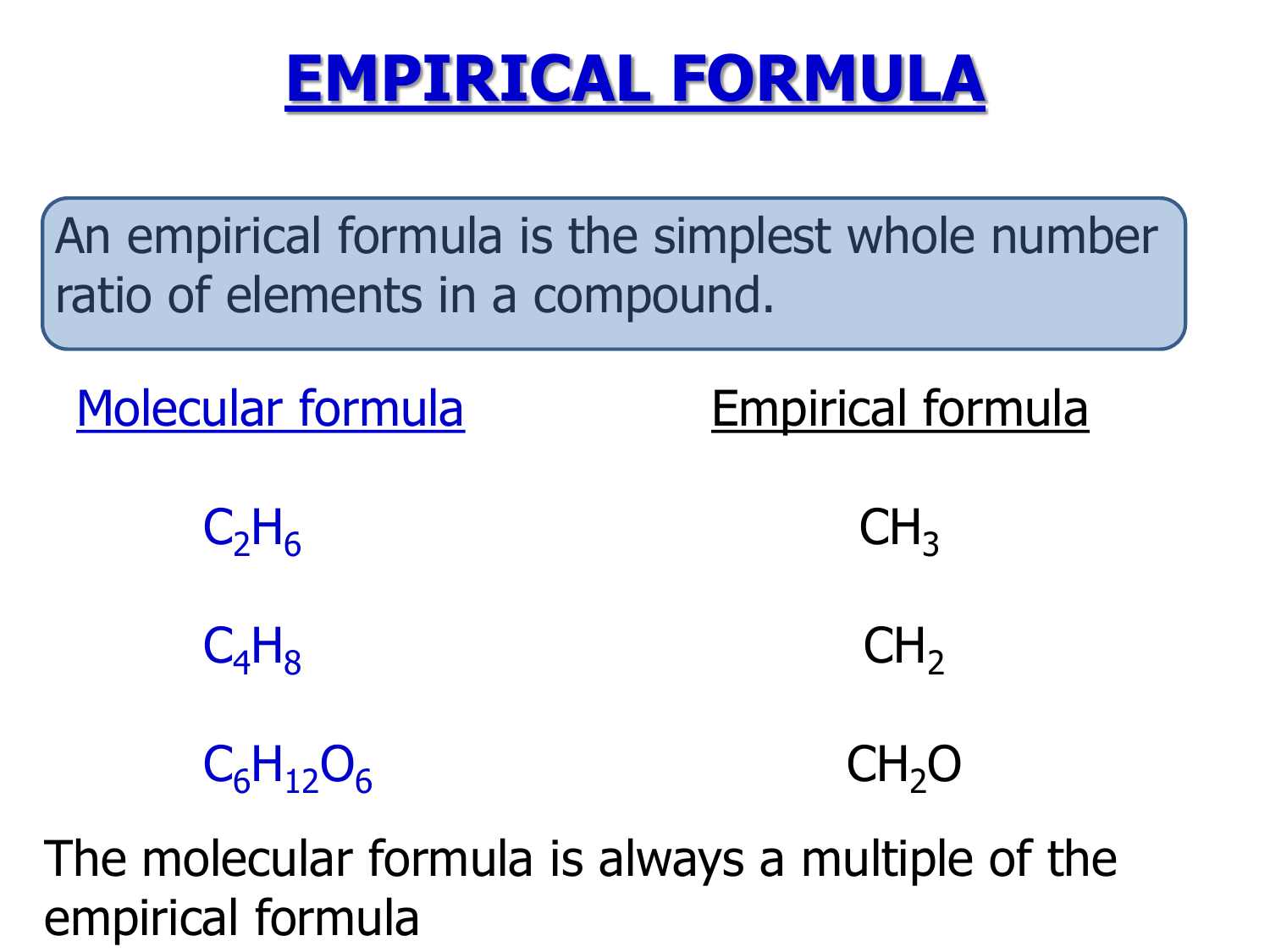
Empirical Formula
https://s2.studylib.net/store/data/005717773_1-d1e85f622480769051c23e84ecafa718.png
Aug 2 2022 nbsp 0183 32 The empirical formula of a compound is defined as the formula that shows the ratio of elements present in the compound but not the actual numbers of atoms found in the molecule The ratios are denoted by subscripts next to the element symbols In order to go from the empirical formula to the molecular formula follow these steps Calculate the empirical formula molar mass EFM Divide the molar mass of the compound by the empirical formula molar mass The result should be a whole number or very close to a whole number
Apr 11 2024 nbsp 0183 32 To find the empirical formula of a compound start by multiplying the percentage composition of each element by its atomic mass For example if a compound is 40 92 percent carbon multiply 40 92 by 12 its atomic mass to get 3 4 Molecular formulas are derived by comparing the compound s molecular or molar mass to its empirical formula mass As the name suggests an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula
More picture related to What Is The Empirical Formula Of The Compound With The Molecular Formula C18h36

Difference Between Empirical And Molecular Formula Class 11 Chemistry
https://i.ytimg.com/vi/0yp20L7_XzU/maxresdefault.jpg

The Empirical Formula And Molecular Mass Of A Compound Are CH2O And
https://i.ytimg.com/vi/hAu5LTI3tMQ/maxresdefault.jpg

Empirical Vs Molecular Formula
https://sciencenotes.org/wp-content/uploads/2020/08/O5-1024x683.png
Feb 17 2020 nbsp 0183 32 The empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound The molecular formula is the representation of the actual whole number ratio between the elements of the compound This step by step tutorial shows how to calculate the empirical and molecular Sep 16 2014 nbsp 0183 32 The empirical formula or simplest formula of a chemical compound is the simplest ratio of elements that make up the molecule These ratios are denoted by subscripts next to the element symbols This example problem will guide you through the steps to determine the empirical formula of a compound
The formula of a compound expressed as the smallest possible whole number ratio of subscripts of the elements in the formula Associated with the empirical formula definition is the one for quot molecular formula quot the formula of a compound in which the subscripts give the actual number of each element in the formula The empirical formula of a compound gives the simplest ratio of the number of different atoms present whereas the molecular formula gives the actual number of each different atom present in a molecule
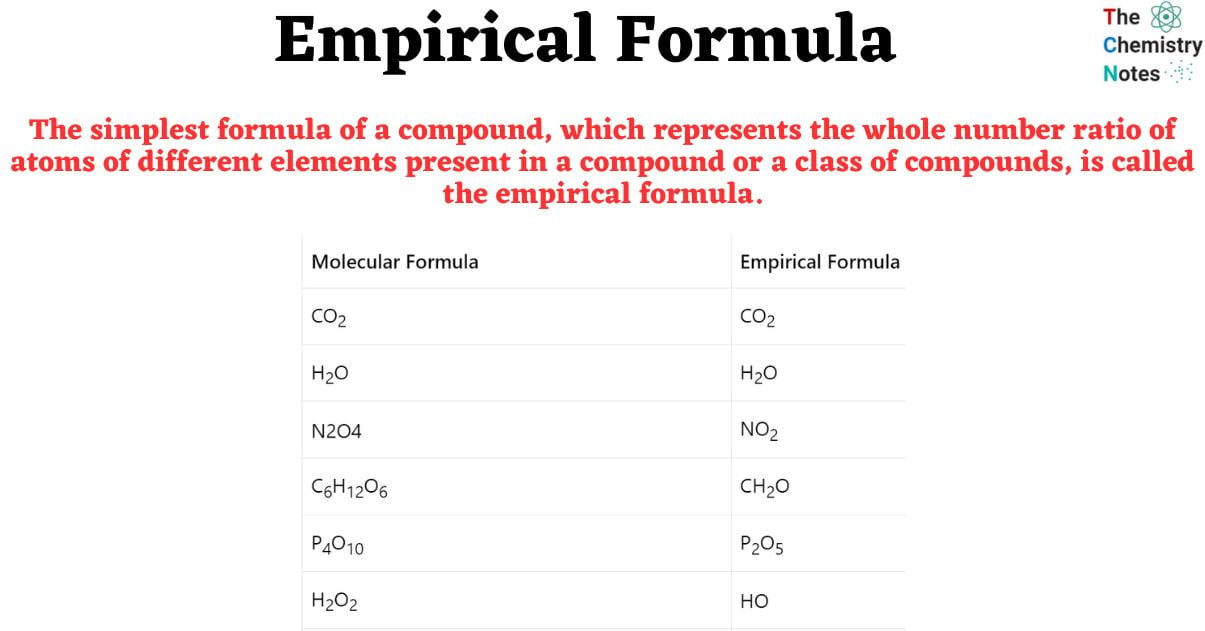
Empirical Formula Definition Calculation
https://scienceinfo.com/wp-content/uploads/2023/08/The-simplest-formula-of-a-compound-which-represents-the-whole-number-ratio-of-atoms-of-different-elements-present-in-a-compound-or-a-class-of-compounds-is-called-the-empirical-formula.jpg

Molecular Formula And Empirical Formula Worksheet Printable Word Searches
https://i2.wp.com/viziscience.com/wp-content/uploads/2017/03/empirical-formula2-1.png
What Is The Empirical Formula Of The Compound With The Molecular Formula C18h36 - Molecular formulas are derived by comparing the compound s molecular or molar mass to its empirical formula mass As the name suggests an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula