What Is The Electronic Configuration Of Oxygen Atomic Number 8 Oxygen is the eighth element with a total of 8 electrons In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital Since 1s can only hold two electrons the
Apr 30 2024 nbsp 0183 32 Oxygen is the 8th element of the periodic table and its atomic number is 8 which means it has 8 electrons The last shell of oxygen has 6 electrons When it gains two electrons turns into an oxide ion O 2 The Jan 21 2021 nbsp 0183 32 Full Electron Configuration For Oxygen Oxygen s Atomic no 8 So the configuration is 1s22s22p4
What Is The Electronic Configuration Of Oxygen Atomic Number 8

What Is The Electronic Configuration Of Oxygen Atomic Number 8
https://i.ytimg.com/vi/46NC3qYPiaI/maxresdefault.jpg

Electronic Configuration Of A Atom Atomic Structure Electronic
https://i.ytimg.com/vi/_XYobUBncHY/maxresdefault.jpg
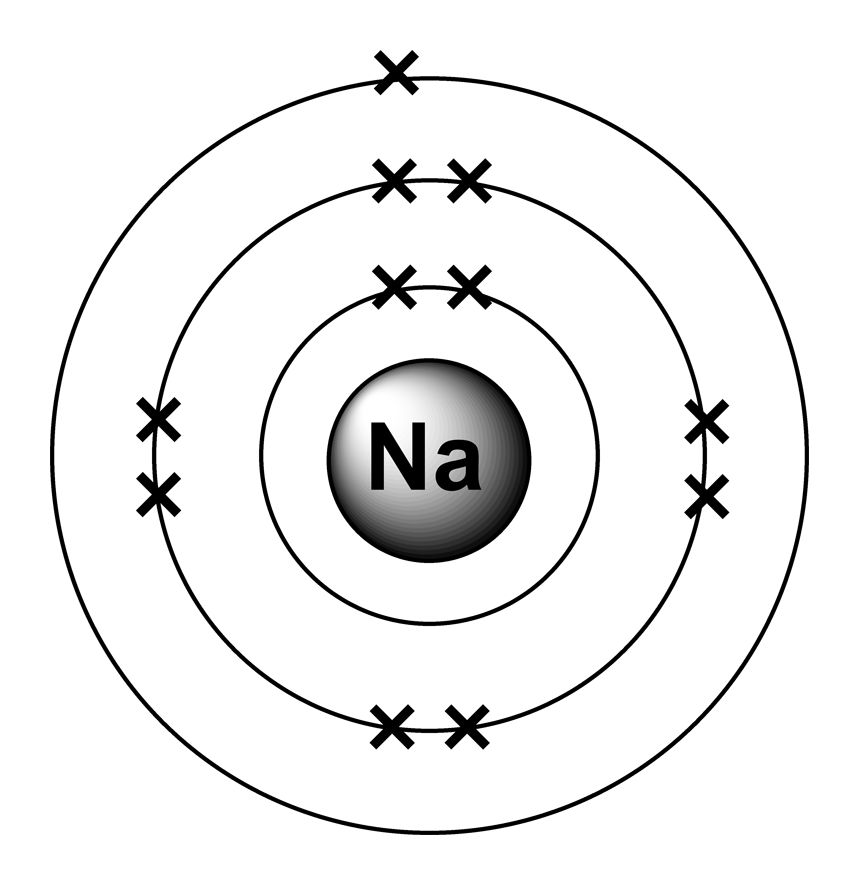
Electron Configurations
http://www.benjamin-mills.com/teaching/chemistry/GCSE/electron-configurations/full-size-images/11-Na-sodium-electron.png
Jul 7 2024 nbsp 0183 32 The atomic number of oxygen is 8 which means its atom has eight electrons outside the nucleus To write the electron configuration for oxygen the first two electrons enter the 1s Oct 12 2023 nbsp 0183 32 Oxygen Electron configuration using the Aufbau Principle An oxygen atom is a neutral atom that has 8 atomic numbers which imply it has a total of 8 electrons As per the Aufbau rule the electrons will be filled into 1s
Oxygen is a chemical element of the periodic table with chemical symbol O and atomic number 8 with an atomic weight of 15 999 u and is classed as nonmetal and is part of group 16 oxygen Nov 13 2020 nbsp 0183 32 Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure The chemical symbol for Oxygen is O Electron configuration of Oxygen is He 2s2 2p4 Possible
More picture related to What Is The Electronic Configuration Of Oxygen Atomic Number 8

Ununseptium JungleKey fr Wiki
http://dic.academic.ru/pictures/wiki/files/101/electron_shell_117_ununseptium.png

Diagramatic Sketch Of Electronic Configuration Of Magnesium atomic
https://hi-static.z-dn.net/files/d58/d165eb1d9f25c8e3d5b23e3a95106e65.jpg
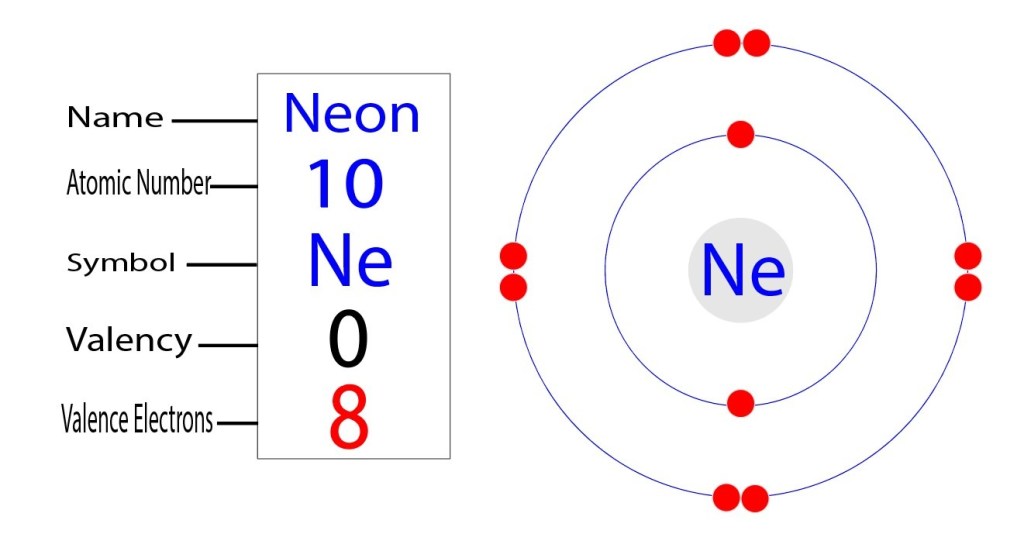
Neon Electron Configuration Number Periodic Table Element
https://iperiodictable.com/wp-content/uploads/2022/02/Neon-Electron-Configuration-Number.jpeg
Electron configuration of oxygen Oxygen is presented as a chemical element which in the periodic table is distinguished by its atomic number 8 and is represented by the symbol O Electronic configuration of the Oxygen atom 1s 2 2s 2 2p 4 Reduced electronic configuration O He 2s 2 2p 4
The atomic number of oxygen is 8 which means it has 8 electrons Now it is possible to find the orbital notation of oxygen very easily through electron configuration That is the orbital notation of oxygen is 1s 2 2s 2 2p 4 What is Oxygen has an atomic number of 8 which means it has 8 electrons The orbitals in oxygen are filled following the Aufbau principle and the Pauli exclusion principle Oxygen has 2 electrons in

Insturctions Carbon Atoms Have Four Valence Electrons Oxygen Atoms
https://us-static.z-dn.net/files/d4d/c434f06d7eebdd0b355b935ba154bb40.png

Oxygen Electron Configuration O With Orbital Diagram
https://periodictable.me/wp-content/uploads/2018/09/Valence-Electrons-are-in-Oxygen-1024x1024.png
What Is The Electronic Configuration Of Oxygen Atomic Number 8 - Oxygen has an atomic number of 8 which means it has 8 electrons The electronic configuration of oxygen is 1s2 2s2 2p4 indicating that it has two electrons in the 1s orbital two electrons in