What Is Redox Reaction With Example Class 10 Ncert Jul 3 2023 nbsp 0183 32 Redox Reaction Those reactions in which oxidation and reduction occurs simultaneously are called Redox Reaction Oxidising Agent The substance which give oxygen
Jun 7 2023 nbsp 0183 32 After learning oxidation and reduction reactions we must know what is redox reaction So today we will learn the definition and examples of redox reactions in detail When oxidation and reduction occur simultaneously in a given chemical reaction it is known as a redox reaction BaSO 4 4C BaS 4CO The reaction in which simultaneous oxidation and reduction take place is called a redox reaction Cu
What Is Redox Reaction With Example Class 10 Ncert

What Is Redox Reaction With Example Class 10 Ncert
https://i.ytimg.com/vi/HEhEuTbrTvo/maxresdefault.jpg

Oxidation And Reduction Reactions Basic Introduction YouTube
https://i.ytimg.com/vi/dF5lB7gRtcA/maxresdefault.jpg

Define Redox Reaction With Example CLASS 10 REDOX REACTIONS
https://i.ytimg.com/vi/50GES2LjFAU/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGFYgVihlMA8=&rs=AOn4CLACmRA0DUG4Q2y3qqlmxN2CFOl_Pg
Apr 13 2025 nbsp 0183 32 List three everyday examples of redox reactions and explain the electron transfer in each case This tests your ability to apply theoretical concepts to practical situations Apr 23 2025 nbsp 0183 32 In this video we cover Redox Reactions Corrosion and Rancidity as per the NCERT syllabus We ve broken down each concept in an easy to understand way with real life
Oxidation of carbohydrates lipids etc into CO 2 and H 2 O to produce energy in the living organisms All oxidation reactions are accompanied by reduction reactions and vice versa Jul 2 2024 nbsp 0183 32 Consider the following examples of redox reactions Reaction Between Zinc Oxide and Carbon ZnO C rightarrow Zn CO Carbon is oxidized to carbon monoxide Zinc oxide is reduced to zinc Reaction Between
More picture related to What Is Redox Reaction With Example Class 10 Ncert

Difference Between Oxidation And Reduction Redox Reaction Class 11
https://i.ytimg.com/vi/fpWNl8VgtAk/maxresdefault.jpg
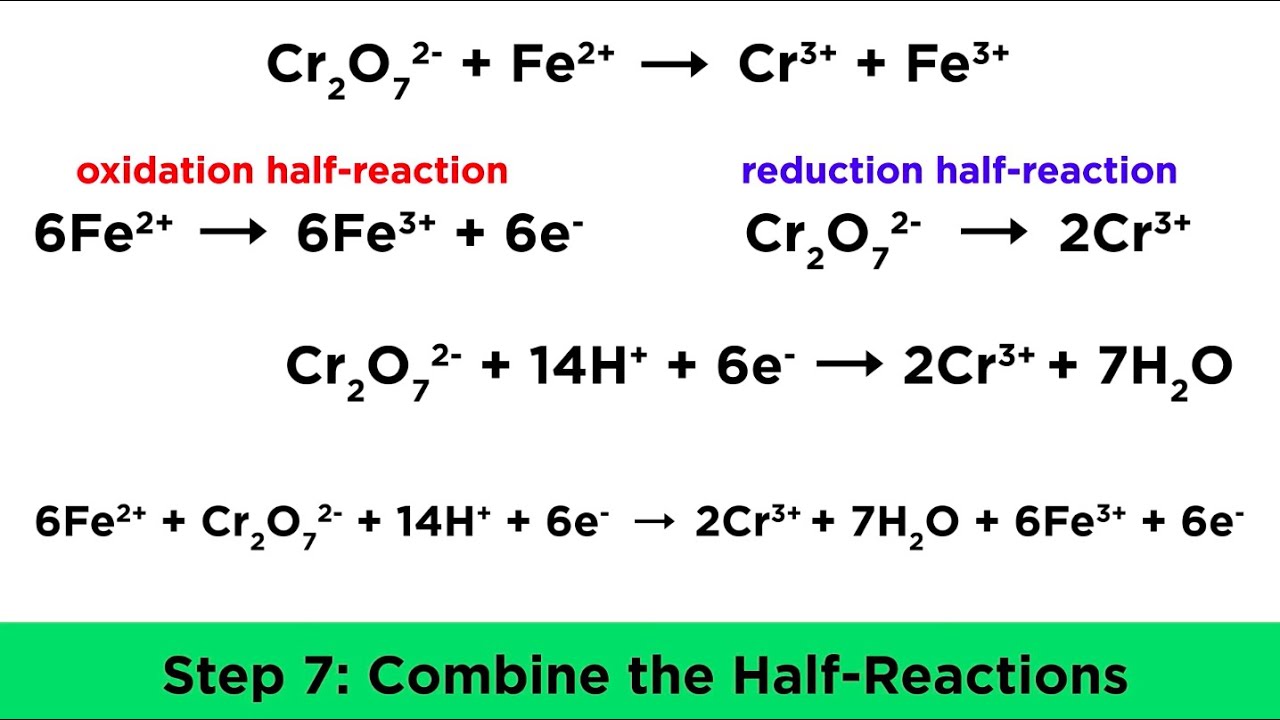
Balancing Redox Reactions In Acidic And Basic Conditions YouTube
https://i.ytimg.com/vi/N6ivvu6xlog/maxresdefault.jpg

Oxidation And Reduction Worksheet Redox Reactions Chemistry
https://i.pinimg.com/736x/4a/85/fc/4a85fc14bebd8bff07f09ea722fed418--redox-reactions-chemistry-teaching-chemistry.jpg
Thus we can define redox reaction as a chemical reaction in which the electrons are transferred between two reactants participating in it This electron transfer can be identified by observing Feb 18 2025 nbsp 0183 32 Redox Reactions Class 10 Science A reaction in which oxidation and reduction occur simultaneously is called a redox reaction Oxidation The process of losing electrons or
Redox is a chemical reaction in which the oxidation states of atoms are changed Any such reaction involves both a reduction process and a complementary oxidation process two key Mar 11 2025 nbsp 0183 32 Master Chemical Reactions and Equations Class 10 with detailed notes MCQs flashcards and Q amp A Perfect for NCERT exam preparation and quick revision

An Oxation Diagram With Two Arrows Pointing To Each Other
https://i.pinimg.com/originals/d6/27/85/d62785aa398bcb9a97ff27d482377640.jpg

Give One Example Of A Redox Reaction Indicate The Oxidizing And
https://hi-static.z-dn.net/files/d27/33f256d75f34c7c95381845f62270936.jpg
What Is Redox Reaction With Example Class 10 Ncert - Jul 2 2024 nbsp 0183 32 Consider the following examples of redox reactions Reaction Between Zinc Oxide and Carbon ZnO C rightarrow Zn CO Carbon is oxidized to carbon monoxide Zinc oxide is reduced to zinc Reaction Between