What Is Rate Of Reaction Jun 10 2018 nbsp 0183 32 Both of these factors increase the frequency of successful collisions and hence increases the rate of reaction When a reaction is irreversible this process is straightforward However when dealing with a reversible reaction the manufacturer must also take in to account 1 Whether the forward reaction is exothermic or endothermic
Nov 21 2023 nbsp 0183 32 Reaction rate is the change in concentration of reactants over time or the change in concentration of products over time Units for reaction rates are in terms of M time For faster reactions Jul 19 2015 nbsp 0183 32 Rate of reaction is simply a measurement of chemical activity of a certain reaction Just like any other types of measurement such as length mass and time rate of reaction is playing a major role in our daily life It is crucial to optimize the rate of reaction to obtain the best performance of the reaction As given in the answer by Michael our body system consists lots
What Is Rate Of Reaction

What Is Rate Of Reaction
https://i.pinimg.com/originals/8c/b3/72/8cb372ff2e94c24e189ef180f580914b.png

Calculating Rates Of Reaction 2016 IB Biology YouTube
https://i.ytimg.com/vi/MCG19QHBOnw/maxresdefault.jpg

Measuring Rates Of Reaction 4 Methods GCSE YouTube
https://i.ytimg.com/vi/LstMh4V2PiA/maxresdefault.jpg
Apr 27 2014 nbsp 0183 32 The rate of reaction of quot A quot is quot quot quot A quot t We insert a minus sign to make the rate a positive number We do not need the minus sign if we are working with a product For example the rate of reaction of C is rate of reaction of quot C quot quot C quot t The overall rate of reaction should be the same whichever component we measure Nov 21 2023 nbsp 0183 32 Rate laws illustrate how the rate of a chemical reaction is determined by the concentration of reactants present The general rate of reaction formula for a rate law is eq Rate k A x B y
Aug 26 2014 nbsp 0183 32 The increased number of collisions caused by a higher pressure increases the reaction rate CHANGE THE PARTICLE SIZE OF A SOLID REACTANT Reaction rate depends on collisions If a reactant is a solid grinding it into smaller particles will increase the surface area The more surface area on which collisions can occur the faster the reaction Nov 21 2023 nbsp 0183 32 The rate law equation also called rate law is a mathematical formula for calculating the rate of a reaction expressed as eq r k A x B y eq when the chemical equation is A B eq
More picture related to What Is Rate Of Reaction

Rate Of Reaction And Rate Law Chemistry Class 12 IIT JEE Main
https://i.ytimg.com/vi/3veVBN1zYCo/maxresdefault.jpg

Reaction Rate Vs Rate Constant Quick Differences And Comparison
https://i.ytimg.com/vi/eLr6tjCe7tY/maxresdefault.jpg

AQA GCSE Chemistry 9 1 Topic 6 The Rate And Extent Of Chemical
https://i.ytimg.com/vi/q0cB0DmgXQw/maxresdefault.jpg
Dec 27 2013 nbsp 0183 32 CHEMICAL REACTION RATES The reaction rate of a chemical reaction is the amount of a reactant reacted or the amount of a product formed per unit time Often the amount can be expressed in terms of concentrations or some property that is proportional to concentration For a reaction such as A 2B we could measure either the rate at which B May 31 2014 nbsp 0183 32 This is largely to do with how much of the reactants are present Let s take this equation 2Mg 2HCl gt 2MgCl H 2 At the start of the reaction when we first put magnesium in the hydrochloric acid there is 100 of the magnesium to react with As a result there will be more successful collisions between the reactant particles The more successful collisions the faster
[desc-10] [desc-11]
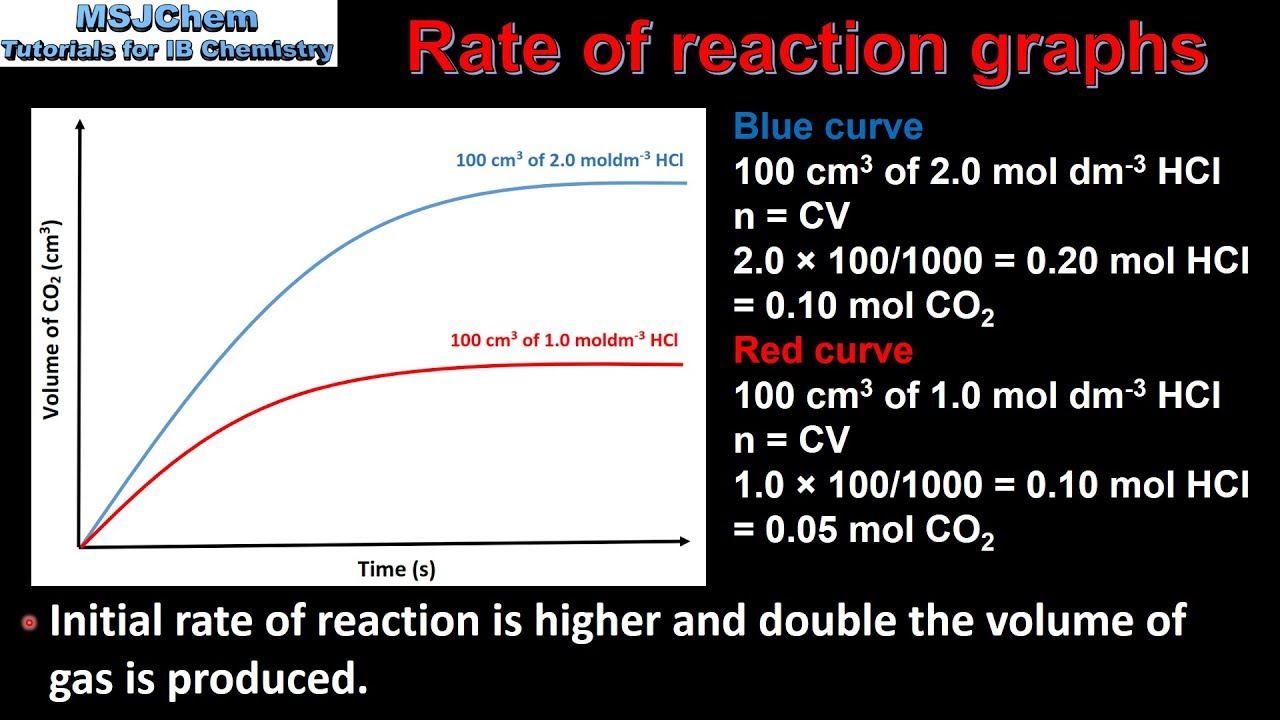
R2 2 1 Analysing Rate Of Reaction Graphs YouTube
https://i.ytimg.com/vi/sk6YqgStN2Q/maxresdefault.jpg

The Rate Equation A Level ChemistryStudent
https://www.chemistrystudent.com/images/A2Physical/kinetics/rateequation1blank.png
What Is Rate Of Reaction - [desc-12]