What Is Polar Covalent And Nonpolar Covalent Dec 3 2017 nbsp 0183 32 The main difference between covalent and polar covalent is that a covalent bond can be either polar or nonpolar whereas a polar covalent bond
In simple terms polar means oppositely charged and non polar means equally charged Covalent bonds can be polar or non polar To understand the difference between polar and non polar bonds it is essential to comprehend Apr 21 2023 nbsp 0183 32 Polar covalent bonds are chemical bonds where electrons are shared unequally between two atoms due to the difference in electronegativity whereas nonpolar covalent bonds
What Is Polar Covalent And Nonpolar Covalent

What Is Polar Covalent And Nonpolar Covalent
https://i.pinimg.com/originals/22/60/37/226037fc7d9eceb5cad416af0648a286.png
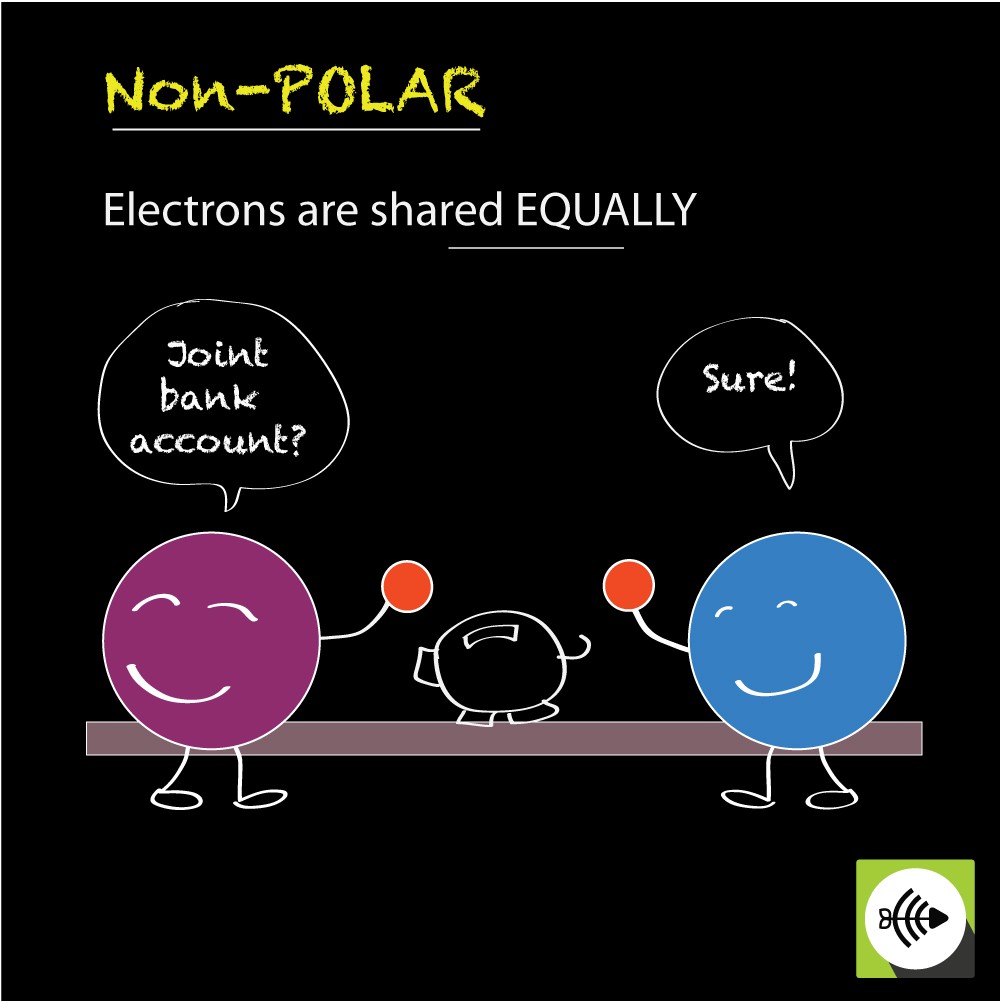
What Is Nonpolar Covalent Bond
https://surfguppy.com/wp-content/uploads/non-polar-1-1000x1002.jpg

Covalent Bond Definition Properties Examples Facts Britannica
https://cdn.britannica.com/04/96904-050-B709B54E/atoms-bond-bonds-electrons-hydrogen-oxygen-atom.jpg
Dec 1 2021 nbsp 0183 32 Polar molecules and nonpolar molecules are the two basic types of molecules Some compounds are unquestionably polar or nonpolar but many have some polarity and lie Feb 26 2018 nbsp 0183 32 Both non polar and polar covalent bonds occur in two different and non metal elements Both classifications also deal with the distribution and sharing of electrons as well as the resulting electronegativity
Nov 8 2020 nbsp 0183 32 Non polar covalent bonds involve the sharing of electrons between atoms with similar electronegativities resulting in a neutral charge distribution polar covalent bonds In chemistry a covalent bond is made of various similar and dissimilar atoms and therefore categorized into polar and nonpolar based on the combining atoms electronegativity
More picture related to What Is Polar Covalent And Nonpolar Covalent
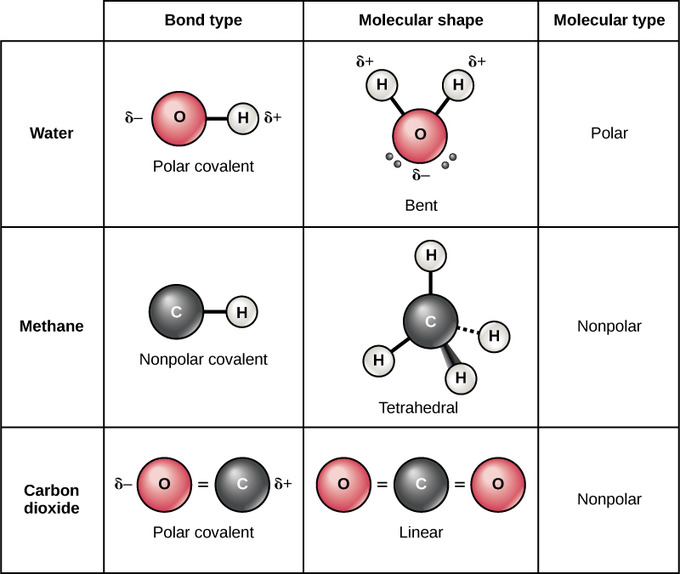
2 9 Atoms Isotopes Ions And Molecules Covalent Bonds And Other
https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/1842/2017/05/26154021/figure-02-01-11.jpeg

Difference Between Polar And Nonpolar Covalent Bond Chemistry
https://i.ytimg.com/vi/VFhmmskR1DA/maxresdefault.jpg
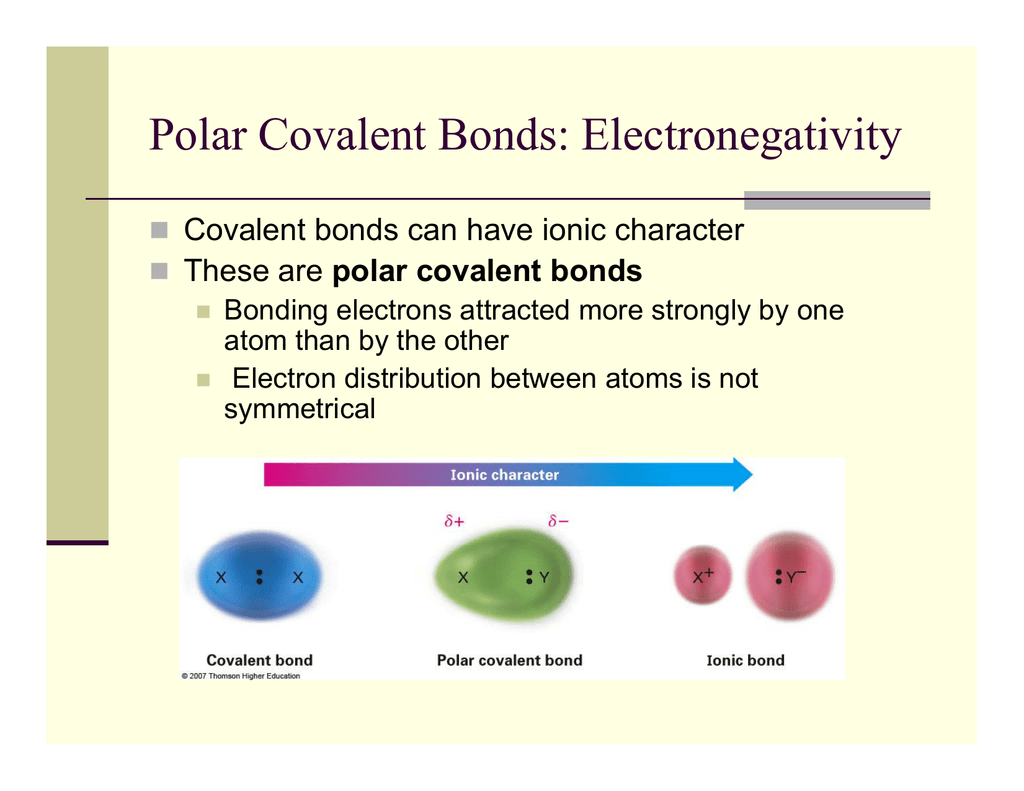
Polar Covalent Bonds Electronegativity
https://s2.studylib.net/store/data/018127556_1-2c904331f07404f3f1695a34db084227.png
Oct 9 2021 nbsp 0183 32 Covalent bonds involve equal sharing of electrons between atoms while polar covalent bonds have unequal electron distribution Nonpolar covalent molecules are electrically Nov 21 2023 nbsp 0183 32 Learn about polar vs nonpolar covalent bonds Discover polar and nonpolar covalent bond examples and examine how to predict polar vs nonpolar bonds Updated
Jul 31 2023 nbsp 0183 32 Understand the key differences between polar and nonpolar covalent bonds Learn about their properties examples and impact on molecular structure Covalent bonds can be non polar or polar and react to electrostatic charges Ionic bonds like those in table salt NaCl are due to electrostatic attractive forces between their positive Na

Nonpolar Covalent Bond Definition And Examples
https://www.chemistrylearner.com/wp-content/uploads/2020/09/Nonpolar-Covalent-Bond-Examples.jpg

Chapter 5 6 Properties Of Polar Covalent Bonds Chemistry LibreTexts
https://chem.libretexts.org/@api/deki/files/39391/679fdbbd60e31f9fd4067f5f482a8f2c.jpg?revision=1
What Is Polar Covalent And Nonpolar Covalent - Understanding polar vs non polar covalent bonds is essential for IB Chemistry SL students Explore key concepts comparisons and applications in this comprehensive guide