What Is Paramagnetic Salt May 15 2019 nbsp 0183 32 O2 is paramagnetic because it has two unpaired electrons Explanation The Lewis structure of O 2 gives a misleading impression It shows that all the electrons in oxygen are
Feb 11 2019 nbsp 0183 32 In contrast paramagnetic and ferromagnetic materials are attracted by a magnetic field 185 Diamagnetism is a quantum mechanical effect that occurs in all materials when it is the Click here to get an answer to your question which set of oxide of nitrogen is paramagentic in monomeric state
What Is Paramagnetic Salt
What Is Paramagnetic Salt
https://i.ytimg.com/vi/bTGVpMECHNw/maxresdefault.jpg

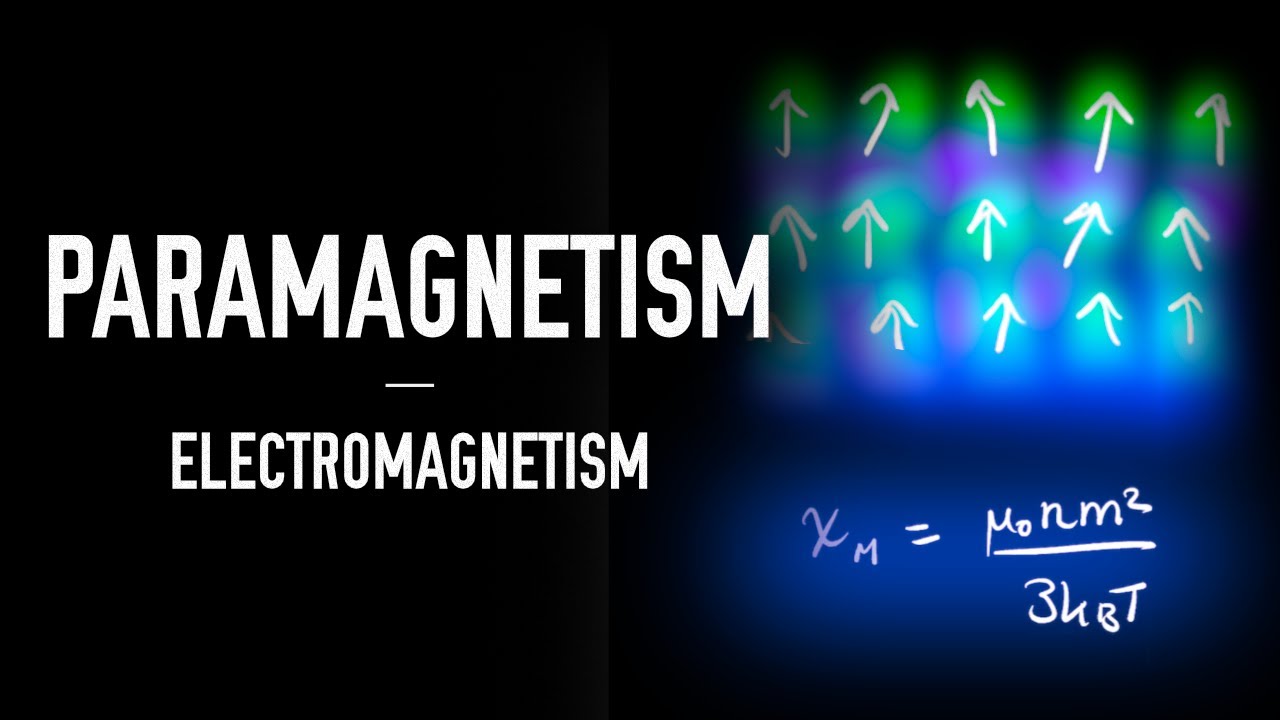
How Does The Magnetic Susceptibility x Of A Paramagnetic Substance
https://i.ytimg.com/vi/PfuuekE9WR0/maxresdefault.jpg

Partition Function For Paramagnetic Salt Statistical Mechanics CSIR
https://i.ytimg.com/vi/OfMLtR3Okh0/maxresdefault.jpg
Aug 11 2019 nbsp 0183 32 KO2 is hence paramagnetic in nature If any of a compound s electrons are unpaired it becomes paramagnetic and if all of its electrons are paired it becomes Aug 21 2021 nbsp 0183 32 Find an answer to your question F2 is diamagnetic while O2 is paramagnetic Explain on the basis of molecular orbital theory
The correct option is A True The given statement is true O2 and O 2 are paramagnetic while O3 and O2 2 are diamagnetic Feb 26 2020 nbsp 0183 32 Answer b carbanion is paramagnetic Explanation Since it contain 7 electrons it contain 4 electron in bonding orbital and 3 electron in antibonding orbital
More picture related to What Is Paramagnetic Salt

What Is Paramagnetic Diamagnetic Ferromagnetic Antiferromagnetic And
https://i.ytimg.com/vi/GXIP6_LX2Ss/maxresdefault.jpg

A Uniform Magnetic Field Gets Modified As Shown In Figure When Two
https://i.ytimg.com/vi/XQadkNgylfI/maxresdefault.jpg
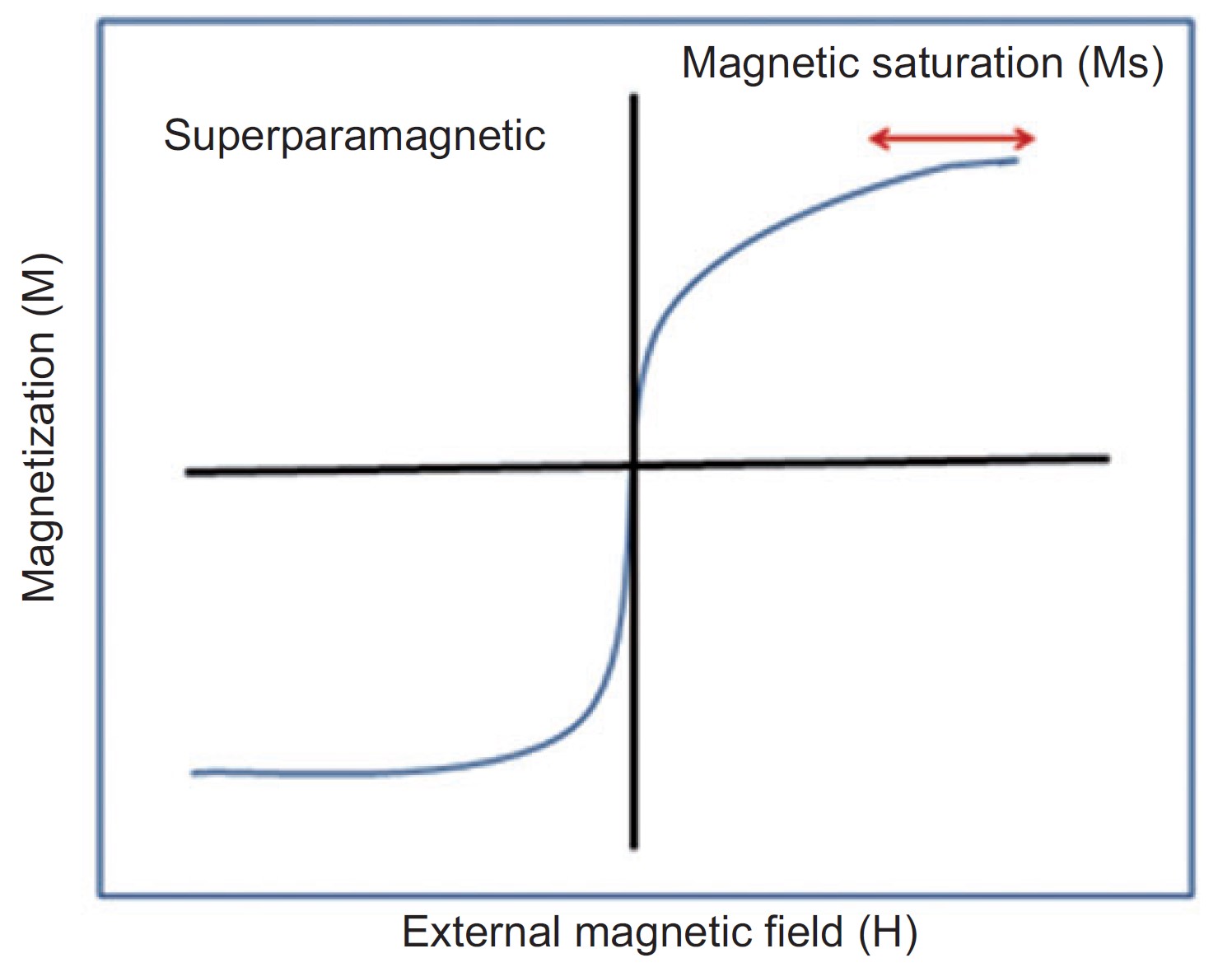
AP Physics 2 Magnetism 15 Magnetic Dipole Moment And 43 OFF
https://www.batterypowertips.com/wp-content/uploads/2023/01/What-are-the-six-types-of-magnetism-Figure-3.jpg
Click here to get an answer to your question compound that is both paramagnetic and coloured is Click here to get an answer to your question how many of the species are paramagneticn2o no no2 o2 no2 no co
[desc-10] [desc-11]
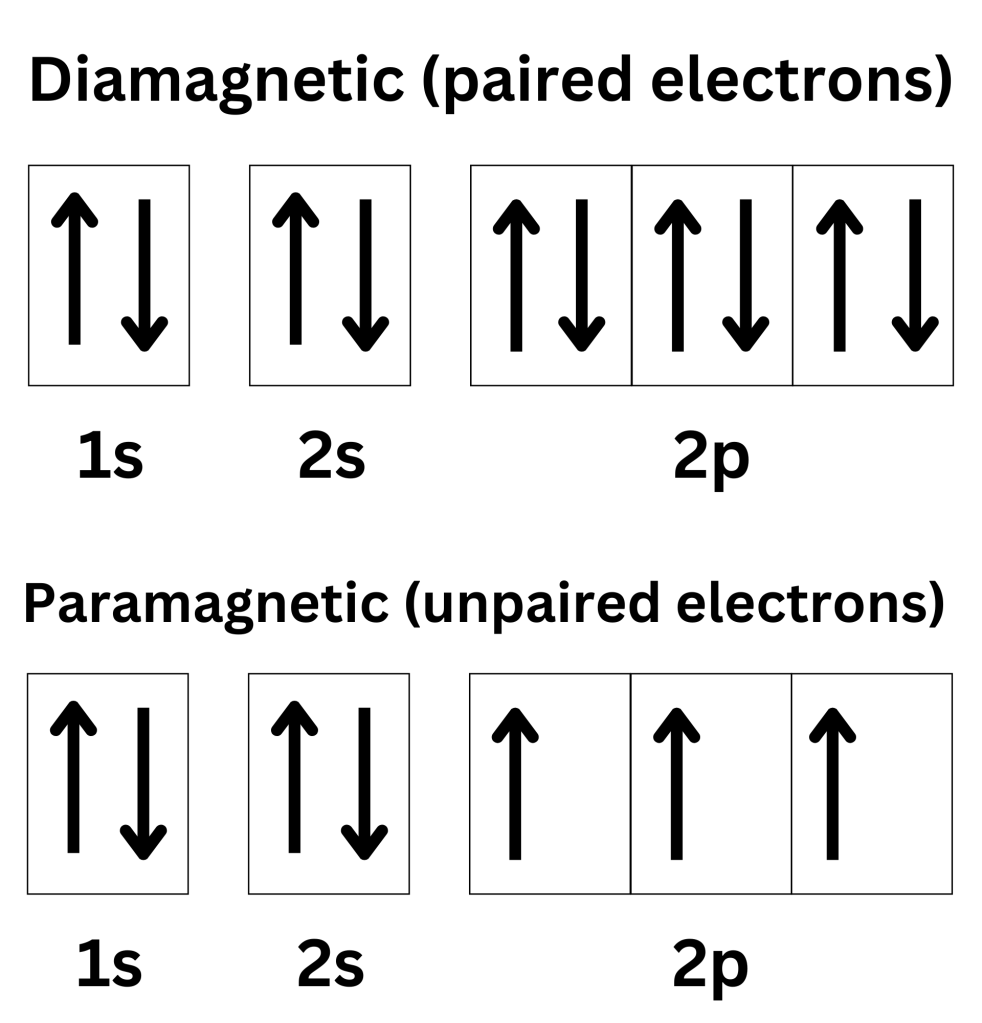
Orbital Diagram Chemistutor
https://chemistutor.org/wp-content/uploads/2024/09/orbital-diagram-paired-vs.-unpaired.png?w=983

Paramagnetism Physics JoVe
https://cloudfront.jove.com/files/media/science-education/science-education-thumbs/14098.jpg
What Is Paramagnetic Salt - [desc-14]