What Is Mole And Avogadro S Number Click here to get an answer to your question calculate the number of electrons in one mole methane
One mole of H 2O and one mole of CO are taken in a 10 litre vessel and heated to 725 K At equilibrium 40 percent of water by mass reacts with carbon monoxide according to the The statement quot There is no difference between one mole and one gram molecule quot is false One mole of any molecule corresponds to Avogadro s number of molecules i e 6 023 215 1023
What Is Mole And Avogadro S Number
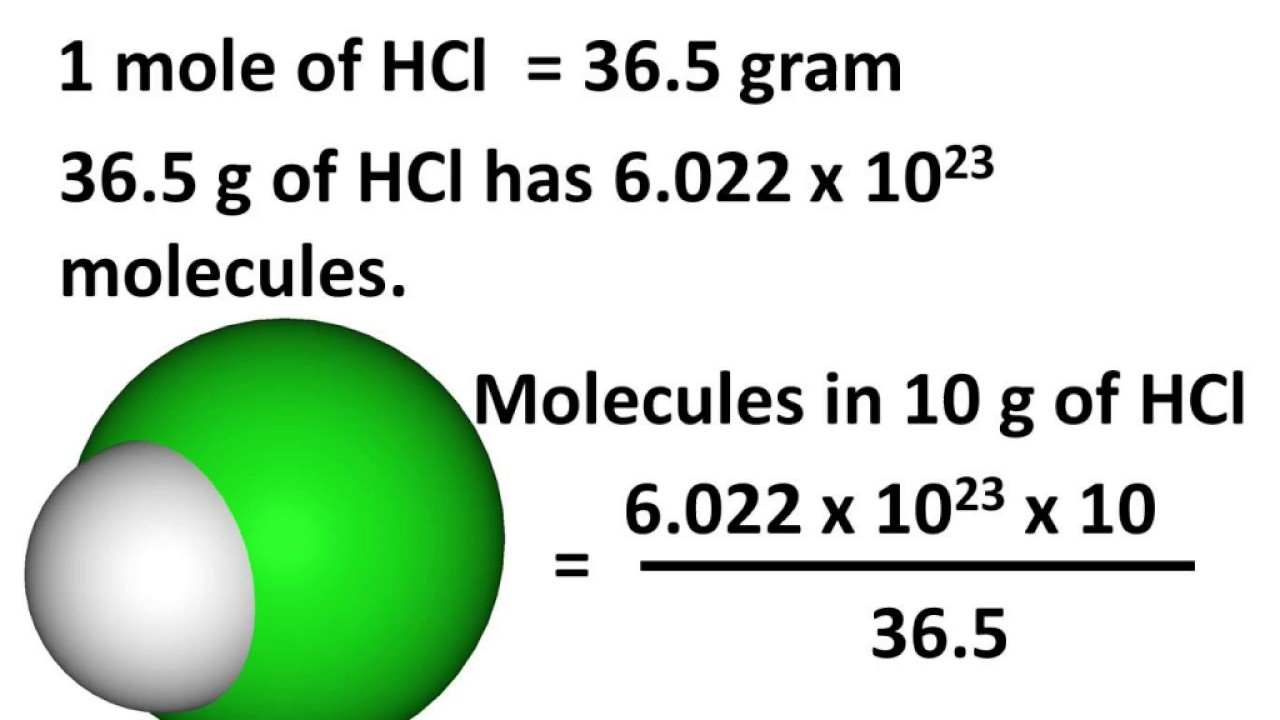
What Is Mole And Avogadro S Number
https://i.ytimg.com/vi/Y2xGT3qh7UE/maxresdefault.jpg

The Mole Definition Avogadro s Number Expii
https://d20khd7ddkh5ls.cloudfront.net/119024616.png

Mole Concept Overview Video Chemistry CK 12 Foundation
https://s3.amazonaws.com/ck12bg.ck12.org/curriculum/107659/thumb_540_50.jpg
One mole of H 2O and one mole of CO are taken in 10 L vessel and heated to 725 K At equilibrium 40 of water by mass reacts with CO according to the equation As given that 4 moles of electrons are transferred to one mole of H N O3 i e the oxidation no of nitrogen in reduction product becomes 1
One mole of an ideal gas at standard temperature and pressure occupies 22 4 L molar volume What is the ratio of molar volume to the atomic volume of a mole of hydrogen Take the size Molality of a solution in aqueous medium is 0 8 Calculate its mole fraction and the percentage by mass of solute if molar mass of solute is 60 View Solution
More picture related to What Is Mole And Avogadro S Number

Avogadros Number Easy Science Easy Science Science Chemistry
https://i.pinimg.com/originals/4e/e6/dd/4ee6ddc9fd6d63a4cf37caa24e646623.png

Avogadro Constant Is The Number 6 02x10 23 Get That In Your Head Ha
https://i.pinimg.com/originals/5e/54/77/5e54772126910b7aa970000a4d58e378.jpg

Mole Day Avogadro The Mole Compound Interest
https://i2.wp.com/www.compoundchem.com/wp-content/uploads/2014/10/The-Mole-2015.png
Question 1 If one mole of carbon atoms weighs 12 grams what is the mass in grams of 1 atom of carbon How many Faraday of electricity is required to reduce 1 mole of M nO 4 ions to M n2 ions
[desc-10] [desc-11]

Avogadro s Number And Mole Concept YouTube
https://i.ytimg.com/vi/nDJhsAwbIJU/maxresdefault.jpg

Avogadro s Constant Surfguppy Chemistry Made Easy Visual Learning
https://surfguppy.com/wp-content/uploads/mole-forehead-1000x1070.jpg
What Is Mole And Avogadro S Number - [desc-12]