What Is Medical Device Validation Jun 17 2020 nbsp 0183 32 Medical products may be subjected to various risks at different stages in the supply chain for example purchasing storage repackaging relabelling transportation and distribution
Explore available job opportunities and apply for a vacancy by creating an online profile Jul 14 2025 nbsp 0183 32 The development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of WHO
What Is Medical Device Validation
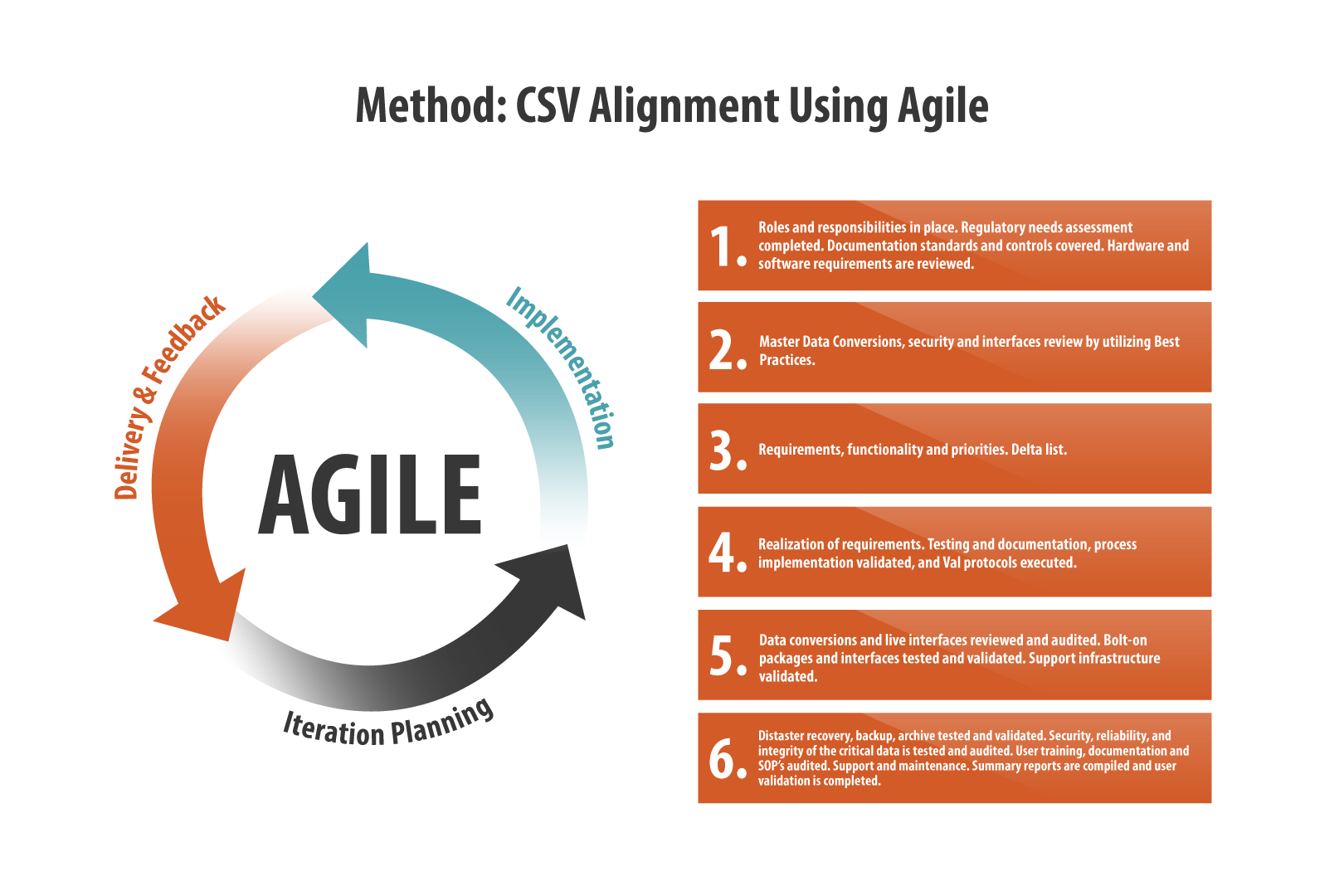
What Is Medical Device Validation
https://www.engilifesciences.com/wp-content/uploads/2017/05/csv-alignment.png

Creating A Testing Plan For Medical Device Manufacturers YouTube
https://i.ytimg.com/vi/u3MjBi9ffSs/maxresdefault.jpg

Difference Between Verification And Validation ISO 9001 Definitions
https://i.ytimg.com/vi/cQIkhR89pUE/maxresdefault.jpg
Jun 12 2014 nbsp 0183 32 This handbook the result of extensive international consultation and collaboration provides comprehensive guidance on safe efficient and environmentally sound methods for WHO provides all interns with medical and accident insurance coverage during the duration of the internship period Insurance coverage before the start date of the internship and after the end
Sep 12 2024 nbsp 0183 32 This WHO Clinical practice guideline for influenza is an update and expansion from the previously published WHO guideline on the clinical management of patients with severe In the Anatomical Therapeutic Chemical ATC classification system the active substances are divided into different groups according to the organ or system on which they act and their
More picture related to What Is Medical Device Validation
Pharma Treasures
http://1.bp.blogspot.com/-R81F95pWlDs/VrN_cmJtvxI/AAAAAAAAATc/9BXmEvg_sFM/s1600/V%2Bmodel.JPG
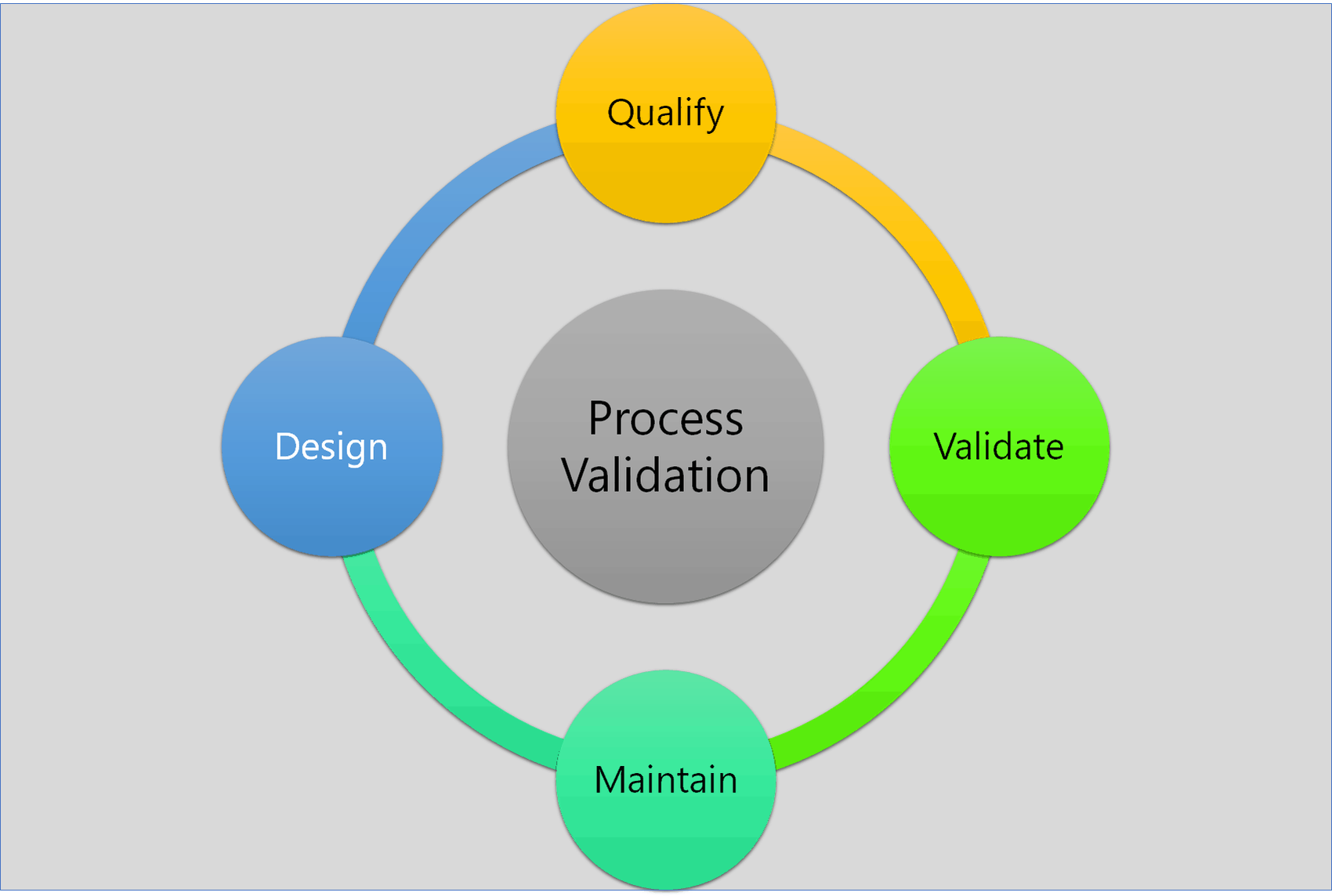
Quality Pharma GxP
https://pharmagxp.com/wp-content/uploads/2020/12/proce.png
Process Validation Record Format Excel PDF Sample
http://www.inpaspages.com/jpg/process_validation_record_02.PNG
Aug 1 2025 nbsp 0183 32 News release WHO designates new WHO Listed Authorities strengthening global access to quality assured medical products 4 August 2025 Jun 30 2021 nbsp 0183 32 Medical devices are used in many diverse settings for example by laypersons at home by paramedical staff and clinicians in remote clinics by opticians and dentists and by
[desc-10] [desc-11]
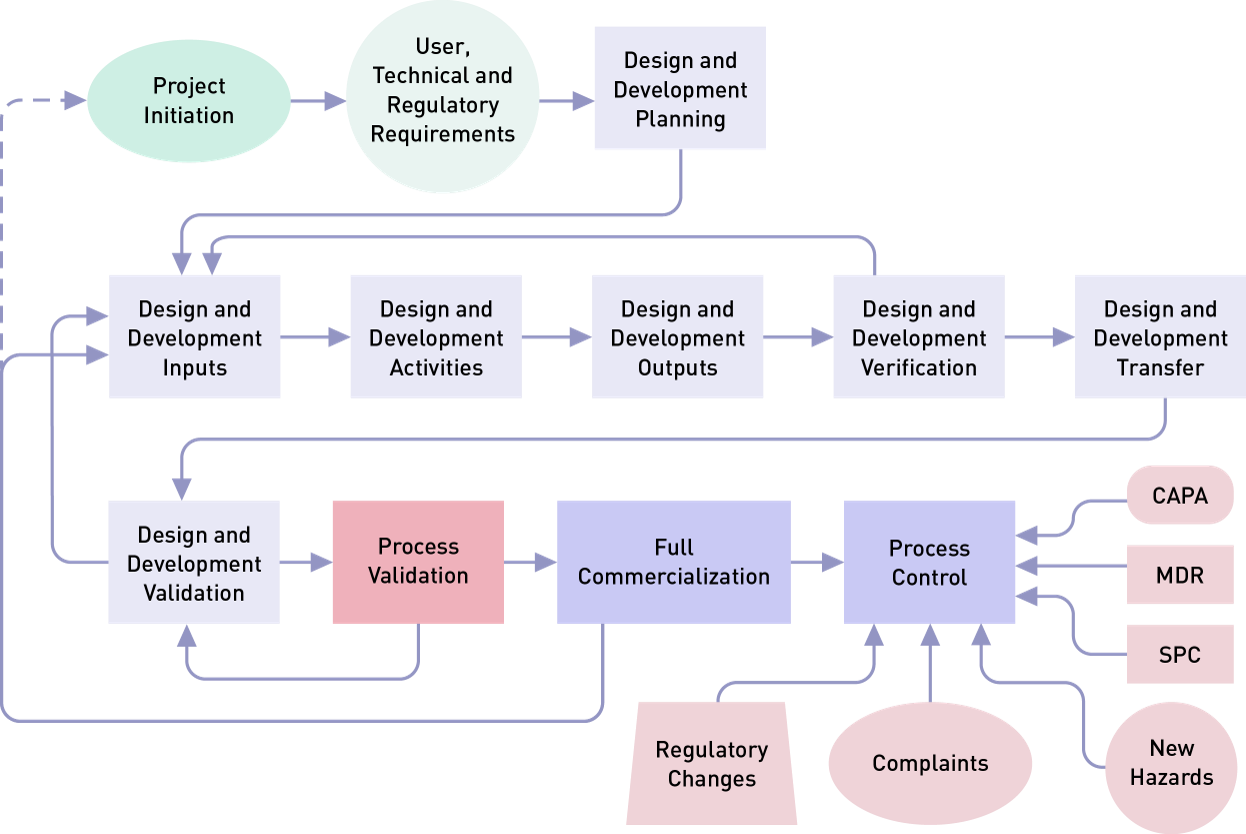
Production Process Glopmath
https://www.orielstat.com/blog/wp-content/uploads/2019/04/Diagram-from-3-5.png

Software Validation Documentation Medical Device Academy
https://medicaldeviceacademy.com/wp-content/uploads/software-validation-documentation.png
What Is Medical Device Validation - Sep 12 2024 nbsp 0183 32 This WHO Clinical practice guideline for influenza is an update and expansion from the previously published WHO guideline on the clinical management of patients with severe