What Is Meant By Instantaneous Rate Of Reaction Feb 7 2019 nbsp 0183 32 Instantaneous rate of reaction is the rate at which the reaction is proceeding at any given time Suppose the value of the term t is very small and tends to zero Now we have an infinitesimally small t which is a very small
The instantaneous rate of reaction 1 The rate of a reaction at a specified or particular point of time 2 It occurs within a short span of time 3 Instantaneous Rate of change Instantaneous Rate d x d t d P d T The average rate of a The instantaneous rate of reaction is the rate at some instant at a particular time Specifically it is defined as the change in concentration of the components of a reaction at an infinitely small time interval
What Is Meant By Instantaneous Rate Of Reaction

What Is Meant By Instantaneous Rate Of Reaction
https://i.ytimg.com/vi/eT1cL46ORdA/maxresdefault.jpg

How To Calculate Instantaneous Rate Of Change From Graph YouTube
https://i.ytimg.com/vi/izCzyujhuYs/maxresdefault.jpg

Theory Of Machines Velocity Analysis By Instantaneous Center Method
https://i.ytimg.com/vi/tRYoDWzvwN4/maxresdefault.jpg
Average rate of a reaction is the change in concentration of reactants or products and the time taken for that change to occur Average rate R t P t R t P t It occurs for a Instantaneous rate of a reaction is defined as the change in the concentration in an infinitesimally small time interval Mathematical Representation of Instantaneous Rate of a Reaction Suppose the value of the term time
Sep 15 2017 nbsp 0183 32 The instantaneous rate of reaction is the slope of the line the tangent to the curve at any time t From SPM Chemistry How do we determine it We draw the best tangent to the line that we can and extend it to The instantaneous reaction rate is the rate at a specific instant during a chemical reaction The average reaction rate corresponds to the slope of the secant that intersects the curve of a concentration graph at any two points
More picture related to What Is Meant By Instantaneous Rate Of Reaction

Theory Of Machines Velocity Analysis By Instantaneous Center Method
https://i.ytimg.com/vi/7lJjk8UqWqY/maxresdefault.jpg
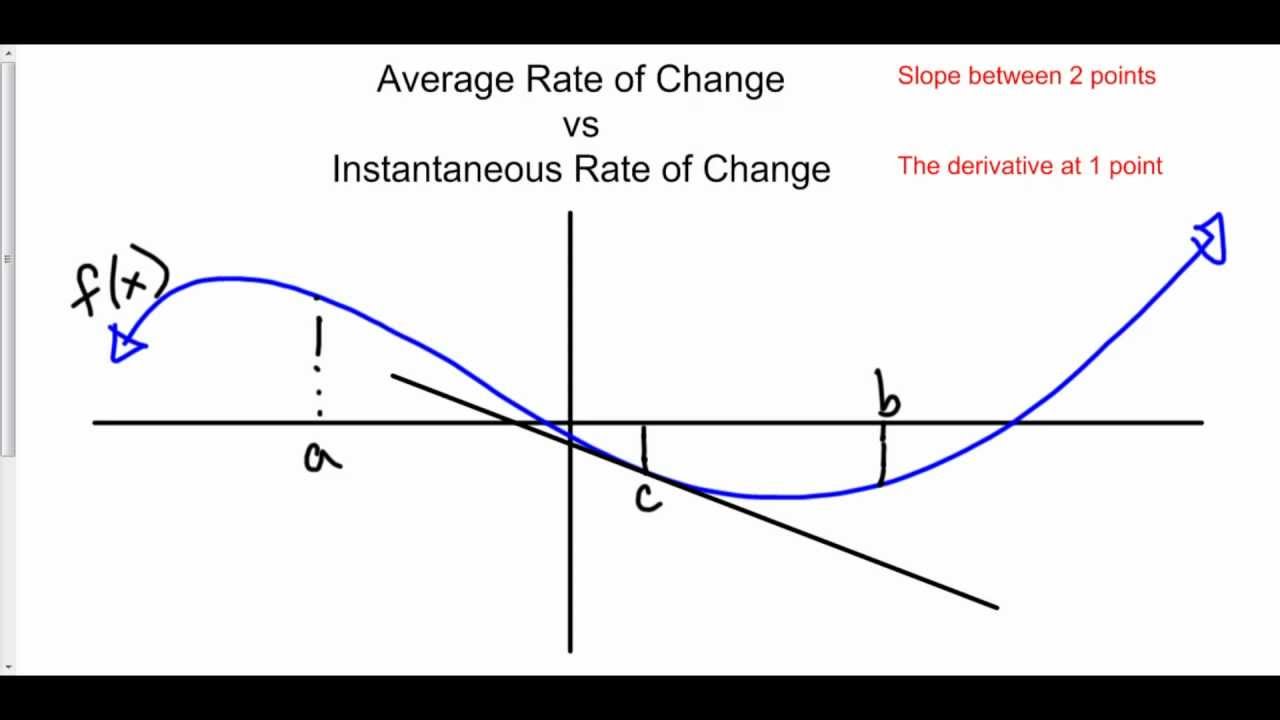
Average Vs Instantaneous Rate Of Change YouTube
https://i.ytimg.com/vi/J7VbhV_yp2U/maxresdefault.jpg

Theory Of Machines Velocity Analysis By Instantaneous Center Method
https://i.ytimg.com/vi/Al5RrMZOUlE/maxresdefault.jpg
Dec 25 2024 nbsp 0183 32 From the Average Rate of Reaction we can understand it as when the change in the time interval is very less i e t 0 then the Rate of Reaction is termed as Instantaneous The instantaneous rate of reaction depends on the change in concentration of an infinity small time interval expressed as the limit or derivation of expression For a particular function the
Instantaneous rate of reaction It may be defined as the change in concentration of a reactant or product of a chemical reaction at a given instant So we can calculate the instantaneous Mar 18 2024 nbsp 0183 32 The reaction rate or rate of reaction is the speed at which a chemical reaction occurs that is how fast the reactants turn into products The rate of chemical reactions varies

Reaction Rates How To Determine The Instantaneous Rate Of Reaction
https://i.ytimg.com/vi/Ra_enwPKw34/maxresdefault.jpg

Instantaneous Velocity In Physics Formula Definition Examples 1
https://i.ytimg.com/vi/6FAFVkl66i4/maxresdefault.jpg
What Is Meant By Instantaneous Rate Of Reaction - Average rate depends upon the change in concentration of reactants or products and the time taken for that change to occur However the average rate cannot be used to predict the rate of