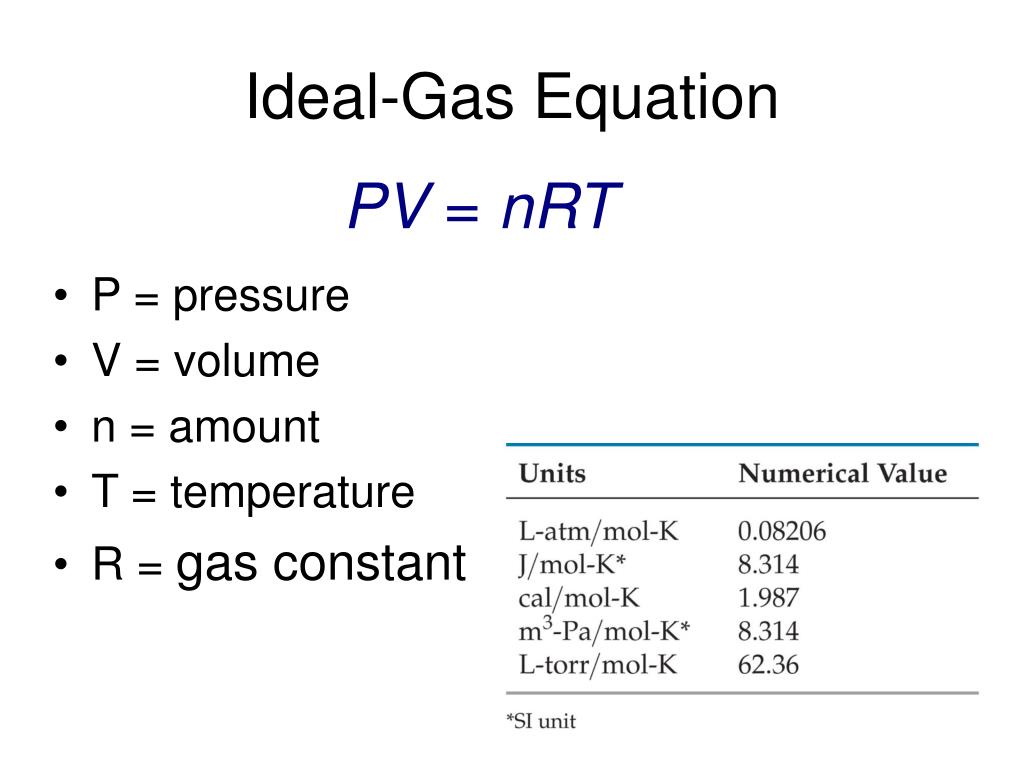
What Is Ideal Equation Jan 30 2023 nbsp 0183 32 The Ideal Gas Equation Before we look at the Ideal Gas Equation let us state the four gas variables and one constant for a better understanding The four gas variables are
The state of an amount of gas is determined by its pressure volume and temperature The modern form of the equation relates these simply in two main forms The temperature used in the equation of state is an absolute temperature the appropriate SI unit is the kelvin The most frequently introduced forms are where The Ideal Gas Equation The ideal gas equation is pV nRT On the whole this is an easy equation to remember and use The problems lie almost entirely in the units I am assuming
What Is Ideal Equation

What Is Ideal Equation
https://d20khd7ddkh5ls.cloudfront.net/ideal_gas_law_formula_2.jpeg
Ideal Gas Law Weather Calculator TasubtitleX
https://image3.slideserve.com/6794964/ideal-gas-equation2-l.jpg

Ideal Gas Law Formula And Examples
https://sciencenotes.org/wp-content/uploads/2022/02/Ideal-Gas-Law-768x512.png
Mar 27 2025 nbsp 0183 32 Ideal gas law relation between the pressure P volume V and temperature T of a gas in the limit of low pressures and high temperatures Mar 12 2025 nbsp 0183 32 Ideal gas a gas that conforms in physical behavior to a particular idealized relation between pressure volume and temperature called the ideal gas law which states that the product of the volume and pressure is proportional to
Jul 7 2023 nbsp 0183 32 The ideal gas law is derived from empirical relationships among the pressure the volume the temperature and the number of moles of a gas it can be used to calculate any of Feb 8 2022 nbsp 0183 32 The ideal gas law is the equation of state for an ideal gas that relates pressure volume gas quantity and absolute temperature Although the law describes the behavior of an ideal gas it approximates real gas behavior
More picture related to What Is Ideal Equation

Ideal Gas Law Worksheet 2 Answer Ideal Gas Law Worksheet PV NRT Use
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/2fafe445515d2d847a7b1ce3a100c705/thumb_1200_1553.png
Formulas For Gas Laws
https://2.bp.blogspot.com/-52PUTTjeHVw/VLu5_NLrJII/AAAAAAAABJQ/VMcC587XWLg/s1600/gas_laws1.jpg
Ideal Body Weight Calculation Tool Gear Up To Fit
https://gearuptofit.com/wp-content/uploads/2019/08/Ideal-Body-Weight-Illustration.jpg
The ideal gas equation is formulated as PV nRT In this equation P refers to the pressure of the ideal gas V is the volume of the ideal gas n is the total amount of ideal gas that is measured in terms of moles R is the universal gas The ideal gas law is a law that explains the state of an ideal gas It establishes a relationship between macroscopic properties like pressure volume temperature and the number of moles Many gases like hydrogen oxygen nitrogen and
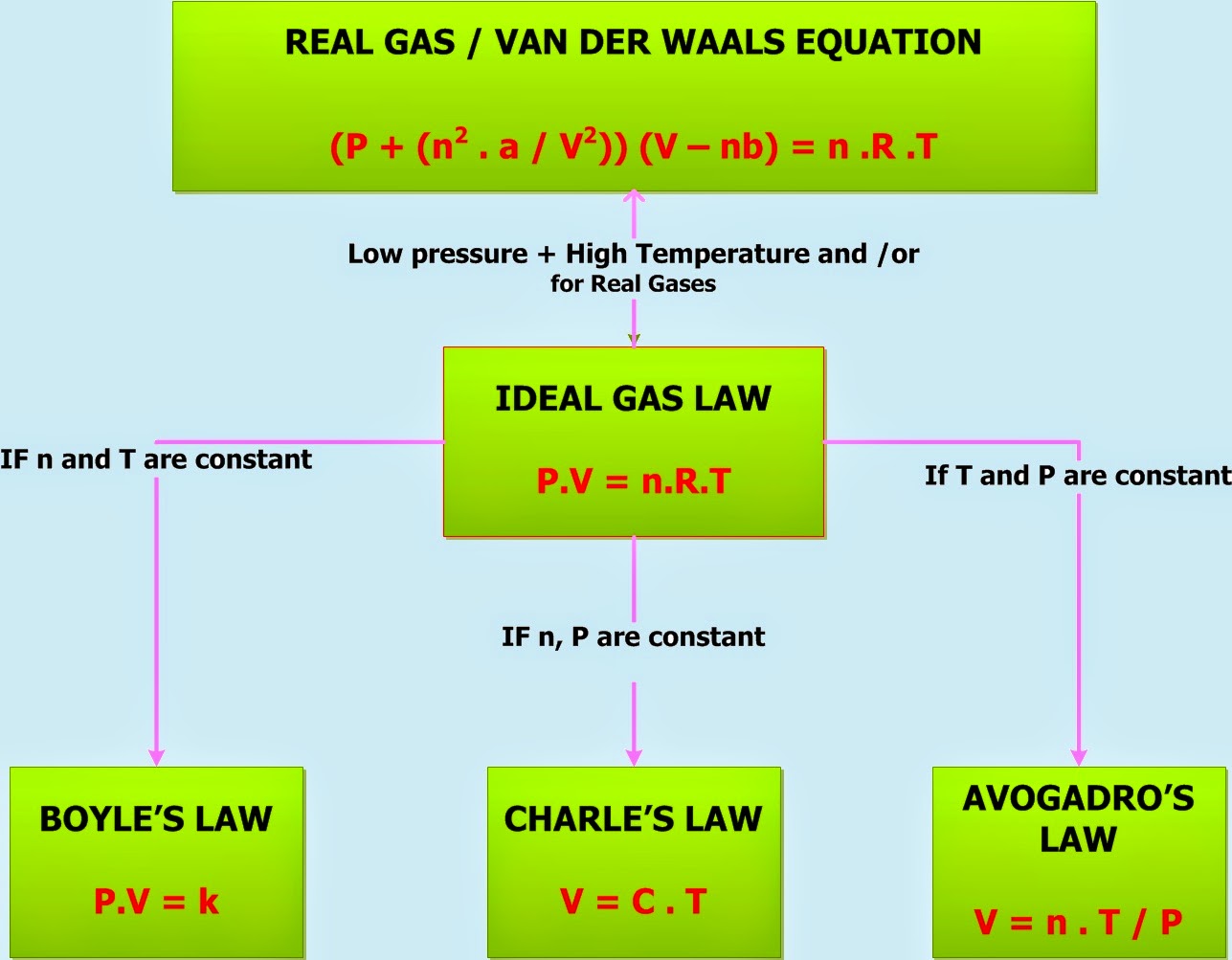
In a perfect or ideal gas the correlations between pressure volume temperature and quantity of gas can be expressed by the Ideal Gas Law The Universal Gas Constant R u is independent Ideal gas assumptions The ideal gas law allows to represent the behavior of gases at low pressure In order to reach a simple form it takes the assumption that there is no interaction in

Ideal Gas Law Statement Characteristics Formula Problems
https://www.chemistrylearner.com/wp-content/uploads/2022/04/Ideal-gas-law.jpg

Math Equation Clipart Cartoon School Background With Some Colorful
https://png.pngtree.com/png-clipart/20230914/original/pngtree-math-equation-clipart-cartoon-school-background-with-some-colorful-doodles-vector-png-image_12151933.png
What Is Ideal Equation - Feb 8 2022 nbsp 0183 32 The ideal gas law is the equation of state for an ideal gas that relates pressure volume gas quantity and absolute temperature Although the law describes the behavior of an ideal gas it approximates real gas behavior