What Is Gay Lussac Law Of Combining Volume Jun 25 2023 nbsp 0183 32 The law of combining volumes was proposed by Gay Lussac at about the same time that Dalton published his atomic theory Shortly thereafter Avogadro suggested the hypothesis that equal volumes of gases contained equal numbers of molecules
The law of Gay Lussac is a variant of the ideal gas law where the volume of gas is held constant The pressure of a gas is directly proportional to its temperature while the volume is kept constant P T constant or Pi Ti Pf Tf are the standard calculations for Gay Lussac s law come to be known as Gay Lussac s law of combining gases The first part of the law says that when gases combine chemically they do so in numerically simple volume ratios Gay Lussac illustrated this part of his law with three oxides of nitrogen
What Is Gay Lussac Law Of Combining Volume
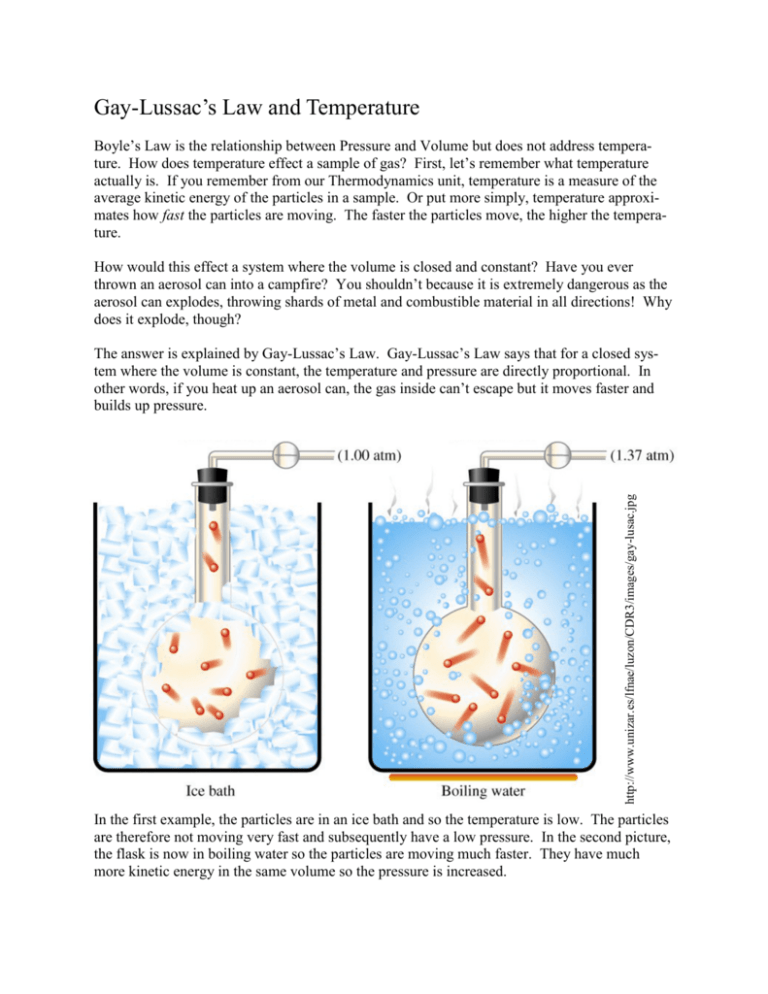
What Is Gay Lussac Law Of Combining Volume
https://s3.studylib.net/store/data/008253142_1-af649895dd8efbe702f9ed16a860bf08-768x994.png

Gay Lussac s Law Of Combining Volume Part 6 YouTube
https://i.ytimg.com/vi/HfKvlYOaRlU/maxresdefault.jpg

Gay Lussac s Law
https://s2.studylib.net/store/data/005360362_1-c3cef59981dfa787cd8938fc0b1fe695-768x994.png
Apr 1 2021 nbsp 0183 32 Gay Lussac s law or Amonton s law states that the absolute temperature and pressure of an ideal gas are directly proportional under conditions of constant mass and volume In other words heating a gas in a sealed container causes its pressure to increase while cooling a gas lowers its pressure Jan 15 2020 nbsp 0183 32 The law of combining volumes is also known as Gay Lussac s law as Gay Lussac described how the pressure of enclosed gas is directly proportional to its temperature circa 1808 Gay Lussac found two volumes of hydrogen and two volumes of oxygen reacted to yield two volumes of water
Aug 17 2023 nbsp 0183 32 What is Gay Lussac s Law Gay Lussac s Law sometimes known as the law of combining volumes is a fundamental principle in the field of chemistry According to Gay Lussac s Law a gas s pressure and temperature are both inversely correlated when kept constant volume and a constant number of moles Law of Combining Volumes of Gases In 1808 Gay Lussac announced what was probably his single greatest achievement from his own and others experiments he deduced that gases at constant temperature and pressure combine in simple numerical proportions by volume and the resulting product or products if gases also bear a simple proportion
More picture related to What Is Gay Lussac Law Of Combining Volume

Law Combining Volumes Gay Lussac Stock Vector Royalty Free 1711030981
https://www.shutterstock.com/shutterstock/photos/1711030981/display_1500/stock-vector-law-of-combining-volumes-gay-lussac-1711030981.jpg

What Is Gay Lussac s Law Its Explanation Applications And
https://learnmechanical.com/wp-content/uploads/2022/09/Picture7-2048x1519.png

What Is Gay Lussac Law Brainly in
https://hi-static.z-dn.net/files/da6/517a85dcb025260fd32eb8e4144db8f2.jpg
Gay Lussac s law of gaseous volumes also known as the law of combining volumes states that the ratio of the volumes of gases involved in a chemical reaction is directly proportional to the ratio of stoichiometric coefficients in the balanced chemical equation provided all gases are at the same temperature and pressure May 6 2024 nbsp 0183 32 Gay Lussac s law states that the pressure exerted by a gas varies directly with the absolute temperature of the gas if the mass of the gas is fixed and the volume is constant i e The pressure exerted by a gas is proportional to the temperature of the gas at constant volume
The law of combining volumes by Gay Lussac states that when different gases react together they do so in terms of volume which bears simple whole number ratio provided that the temperature and pressure of reacting gases remain constant The combined gas law relates pressure volume and temperature of a gas This page titled 8 5 Other Gas Laws is shared under a CC BY NC SA 3 0 license and was authored remixed and or curated by Theodore Chan via source content that was edited to the style and standards of the LibreTexts platform

PPT Gay Lussac s Law Of Combining Volumes Of Gases PowerPoint
https://image3.slideserve.com/6559217/slide1-l.jpg
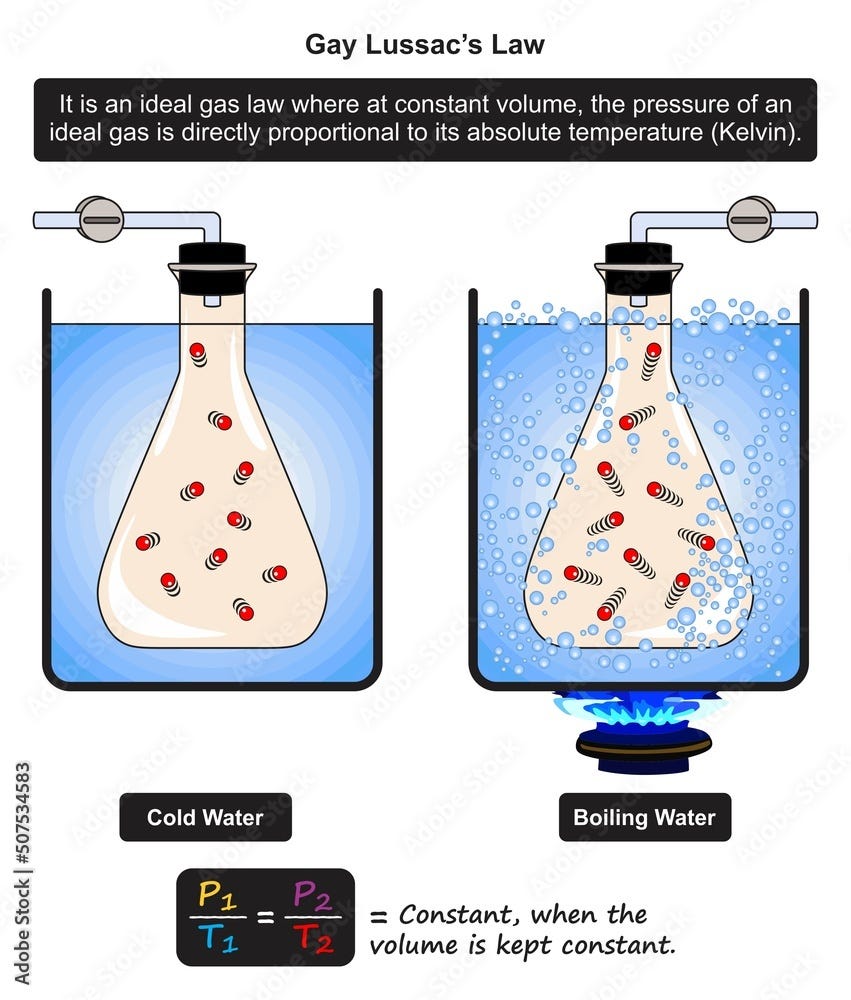
Gay Lussac s Law Teach Chemistry
https://miro.medium.com/v2/resize:fit:851/1*4kz9ayitzGA1w8CxG4vdzQ.jpeg
What Is Gay Lussac Law Of Combining Volume - Law of Combining Volumes of Gases In 1808 Gay Lussac announced what was probably his single greatest achievement from his own and others experiments he deduced that gases at constant temperature and pressure combine in simple numerical proportions by volume and the resulting product or products if gases also bear a simple proportion