What Is Data Management Plan In Clinical Trial Clinical Data Management CDM is a critical phase in clinical research which leads to generation of high quality reliable and statistically sound data from clinical trials This helps to produce a
Jul 17 2024 nbsp 0183 32 A data management plan is a critical tool for ensuring the quality integrity and responsible management of data in a clinical trial A DMP guides Aug 23 2024 nbsp 0183 32 Creating a Data Management Plan DMP for clinical trials is crucial for ensuring data integrity compliance and efficiency throughout the study Here s a comprehensive guide
What Is Data Management Plan In Clinical Trial

What Is Data Management Plan In Clinical Trial
https://s3.amazonaws.com/libapps/accounts/109754/images/research-data-management-cycle3_Curtin.jpg

Database Flowchart Diagram
https://s3.amazonaws.com/thumbnails.venngage.com/template/78526642-150f-4649-91af-aa5cb69b4f76.png

Digital Asset Management Services Digital Asset Management Solutions
https://webwelder.net/wp-content/uploads/2020/12/AdobeStock_251202063-1.jpeg
Aug 28 2023 nbsp 0183 32 A Data Management Plan DMP is a detailed and well structured document that specifies how data will be gathered analyzed stored and Jun 27 2024 nbsp 0183 32 Clinical data management follows a structured series of stages to ensure the integrity and usability of data collected during clinical trials Each stage is crucial for meeting
Jun 13 2024 nbsp 0183 32 Clinical data management system or CDMS is a tool used in clinical research to manage the data of a clinical trial These systems ensure Jan 16 2020 nbsp 0183 32 Clinical data management plans DMPs outline all the data management work needed in a clinical research project This includes the
More picture related to What Is Data Management Plan In Clinical Trial

Clinical Trial Budget Template Download In Excel Google Sheets
https://images.template.net/129153/clinical-trial-budget-template-7xzt6.png

Clinical Study Protocol PowerPoint And Google Slides Template PPT Slides
https://www.collidu.com/media/catalog/product/img/5/c/5ca6d672fc07a8e8b362f858c73f0b9fbc015b9455f99293b9d0984224fc2388/clinical-study-protocol-slide1.png
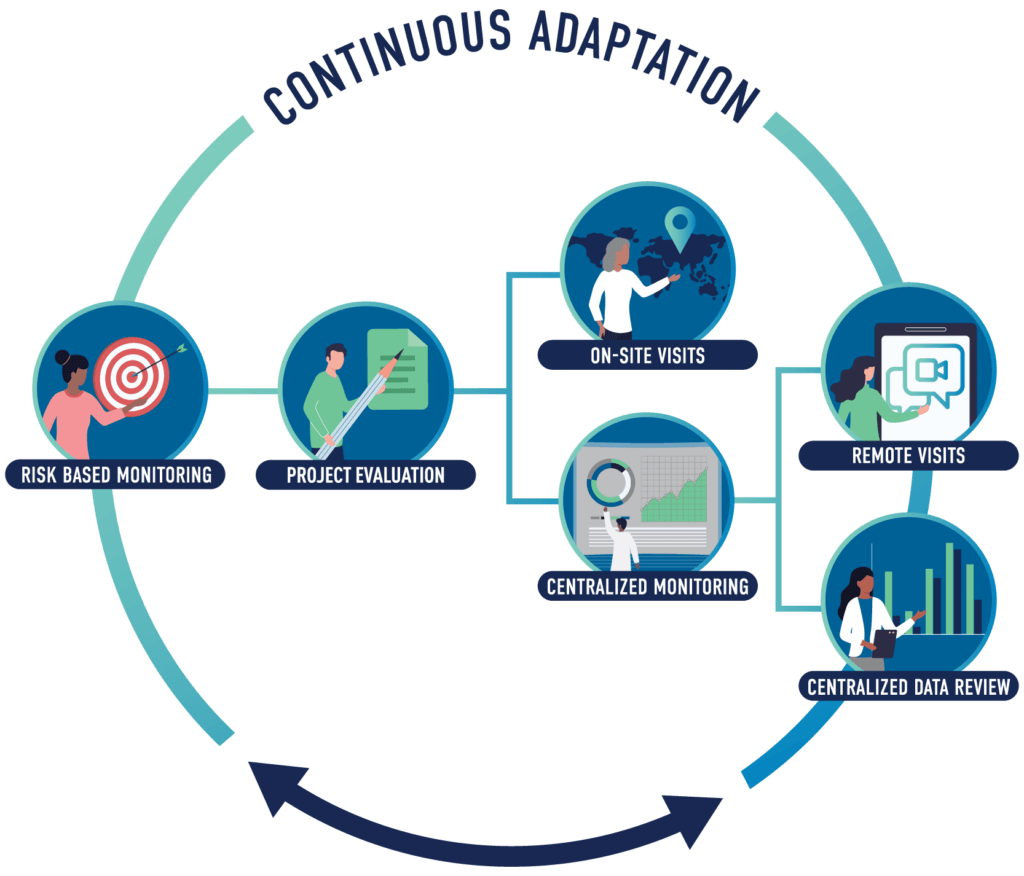
Clinical Monitoring
https://www.medpace.com/wp-content/uploads/2023/03/F2-Continuous-Adaptation-Graphic_0323-1024x876.png
Clinical data management CDM is a critical process in clinical research which leads to generation of high quality reliable and statistically sound data from clinical trials 1 Clinical Dec 5 2023 nbsp 0183 32 What Are the Stages of a Clinical Data Management Cycle The clinical data management cycle is a systematic process that involves the
Apr 15 2025 nbsp 0183 32 Clinical trial data management CTDM is a crucial aspect of any clinical research endeavor It involves the process of collecting organizing A clinical Data Management Plan DMP is a document that describes how data is obtained and handled during all the stages of a clinical trial The DMP includes aspects related to clinical
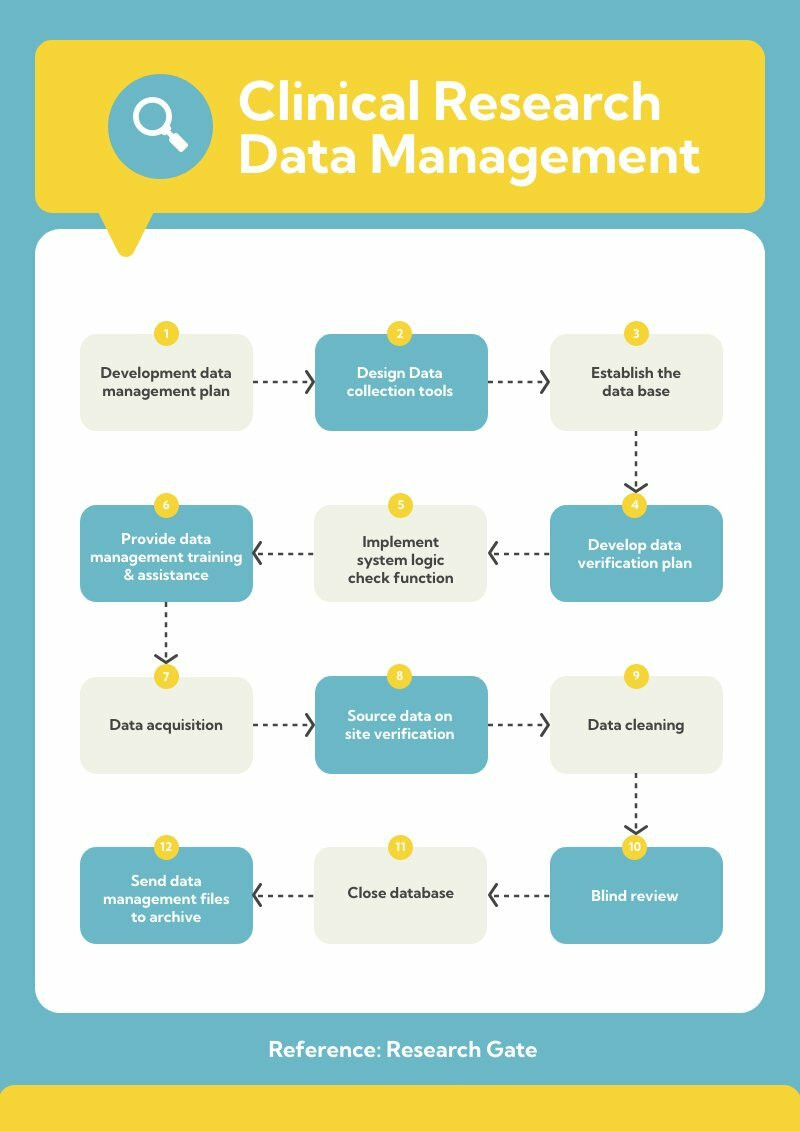
Action Research Flowchart Vrogue co
https://c0.piktochart.com/v2/themes/2721-clinical-research-flowchart/snapshot/large.jpg
Clinical Trial Timeline PowerPoint And Google Slides Template PPT Slides
https://www.collidu.com/media/catalog/product/img/3/c/3c3c34381b3bc663d1cff42ecd7d7c78bc4dbe80931aade18bcb90fcd78e434a/clinical-trial-timeline-slide4.png
What Is Data Management Plan In Clinical Trial - Aug 14 2024 nbsp 0183 32 A Data Management Plan DMP is a document that outlines the procedures tasks and milestones related to data management throughout a clinical trial It covers aspects