What Increase Rate Of Reaction Oct 29 2020 nbsp 0183 32 Mixing helps improve reaction rate Some reactions get their activation energy from light increasing the rate of the chemical reaction Some types of reactions are inherently faster than others Temperature is often the factor that has the greatest effect on reaction rate
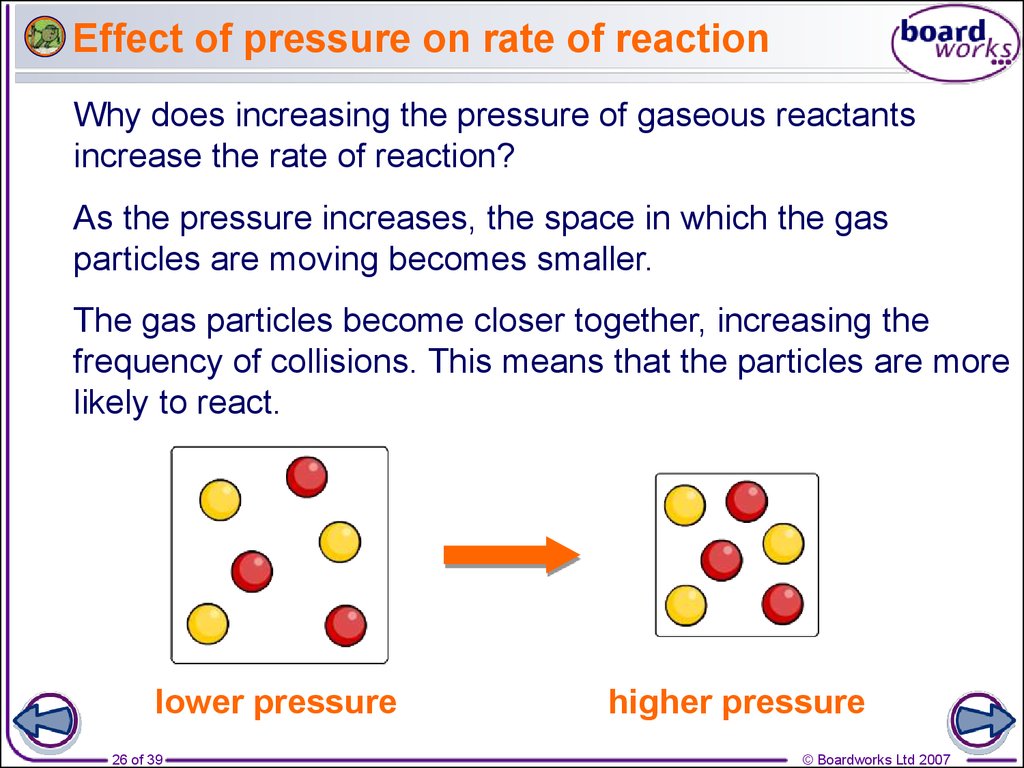
Sep 1 2022 nbsp 0183 32 Three ways to increase the rate of a chemical reaction is by changing factors such as concentration temperature and surface area The equal mass of sugar crystals has a greater surface area than the sugar cube Sep 16 2022 nbsp 0183 32 The rate of reaction is proportional to the number of collisions over time increasing the concentration of either reactant increases the number of collisions and therefore increases the number of successful collisions and the reaction rate
What Increase Rate Of Reaction
What Increase Rate Of Reaction
https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-25.jpg

OCR Gateway B C3 Reaction Rates Particle Size Higher YouTube
https://i.ytimg.com/vi/9Fbwqx1Oy30/maxresdefault.jpg
2 Bachillerato Qu mica Fisica Y Quimica EducaMadrid
http://www.compoundchem.com/wp-content/uploads/2016/02/Factors-Affecting-Rate-of-Reaction.png
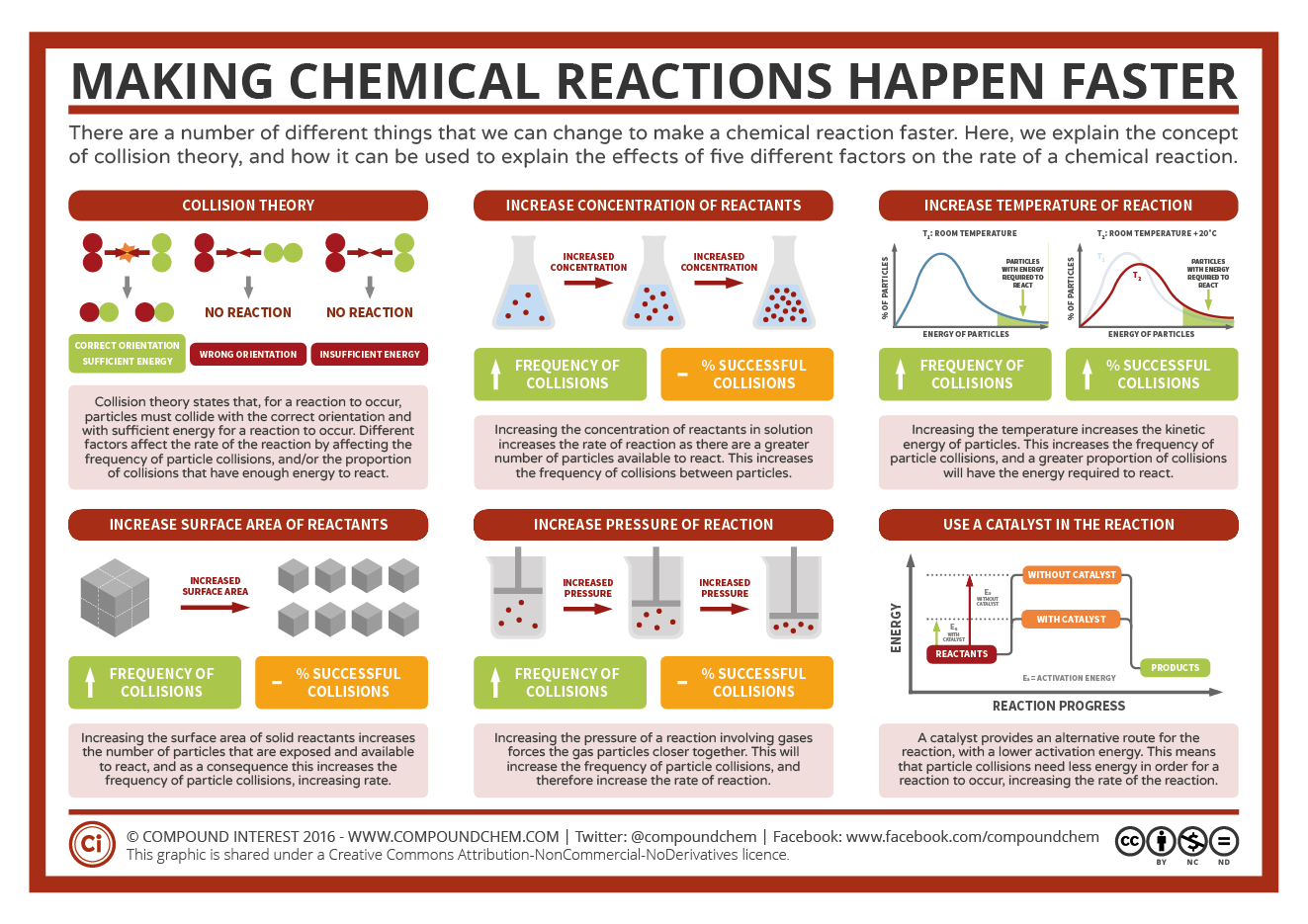
Nov 26 2019 nbsp 0183 32 Mixing reactants increases their ability to interact thus increasing the rate of a chemical reaction The chart below is a summary of the main factors that influence the reaction rate There is typically a maximum effect after which changing a factor will have no effect or will slow a reaction There are four main factors that can affect the reaction rate of a chemical reaction Reactant concentration Increasing the concentration of one or more reactants will often increase the rate of reaction
Chemical reaction rates increase or decrease according to factors including temperature pressure and light A catalyst changes the rate of reaction but is unchanged at the end of the Chemical reaction rates increase or decrease according to factors including temperature pressure and light A catalyst changes the rate of reaction but is unchanged at the end of the
More picture related to What Increase Rate Of Reaction

Rate Determining Step ChemTalk
https://chemistrytalk.org/wp-content/uploads/2023/03/SN1_SN2_comparison.png

Collision Theory
https://www.chemistrystudent.com/images/ASPhysical/kinetics/collisiontheory5.png

Examples Of Reactant
https://d20khd7ddkh5ls.cloudfront.net/concentration_affects_reaction_rate.jpeg
Chemical reaction rates increase or decrease according to factors including temperature pressure and light A catalyst changes the rate of reaction but is unchanged at the end of the reaction Sep 24 2024 nbsp 0183 32 Use our revision notes to understand factors affecting the rate of reaction including temperature concentration surface area and catalysts Learn more
Use a catalyst a catalyst is a substance which increases the rate of a reaction without being used up in the reaction It does this by providing an alternative pathway for the reaction which has a lower activation energy and therefore takes place more quickly There are five main factors that affect the rate of reaction These are During a chemical reaction the five factors mentioned earlier can be changed They can be increased or decreased both of which will have an effect on the rate of reaction Rate of reaction can be increased
Growth Clipart Growth Graph Growth Growth Graph Transparent FREE For
https://webstockreview.net/images/graph-clipart-yellow-4.png

Diagram Of Evaporation
https://d1avenlh0i1xmr.cloudfront.net/a05756c1-ced7-4f94-b64e-383e47b1a757/different-factors-affecting-evaporation-teachoo-01.jpg
What Increase Rate Of Reaction - May 12 2018 nbsp 0183 32 The rate of reaction in general varies directly with changes in the concentration of the reactants When the concentration of all the reactants increases more molecules or ions interact to form new compounds and the rate of reaction increases