What Four Factors Influence The Rate Of Homogeneous Reaction The rate of a homogeneous reaction is influenced by four main factors reactant concentration physical state of the reactants temperature and the presence of a catalyst Higher reactant concentration and greater surface area increase the likelihood of reactant collisions thus speeding up the reaction
Mar 14 2022 nbsp 0183 32 Thus The factors to influence the rate of a homogeneous reaction are Reactant concentration the physical state of the reactants surface area temperature and the presence of a catalyst To learn more about the rate of reaction follow the link There are many factors that influence the reaction rates of chemical reactions include the concentration of reactants temperature the physical state of reactants and their dispersion the solvent and the presence of a catalyst
What Four Factors Influence The Rate Of Homogeneous Reaction

What Four Factors Influence The Rate Of Homogeneous Reaction
https://i.ytimg.com/vi/qIvPSuUsgJU/maxresdefault.jpg
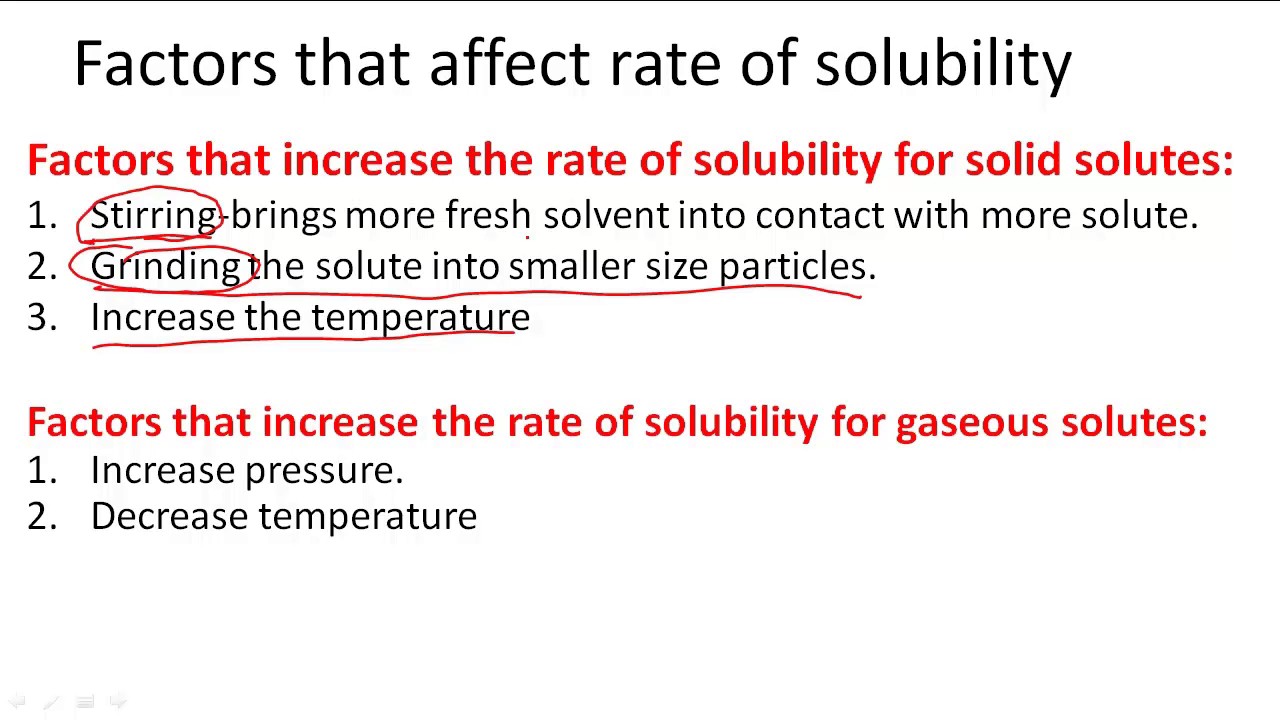
Factors That Affect Rate Of Solubility YouTube
https://i.ytimg.com/vi/pp1Sc2rPOk4/maxresdefault.jpg
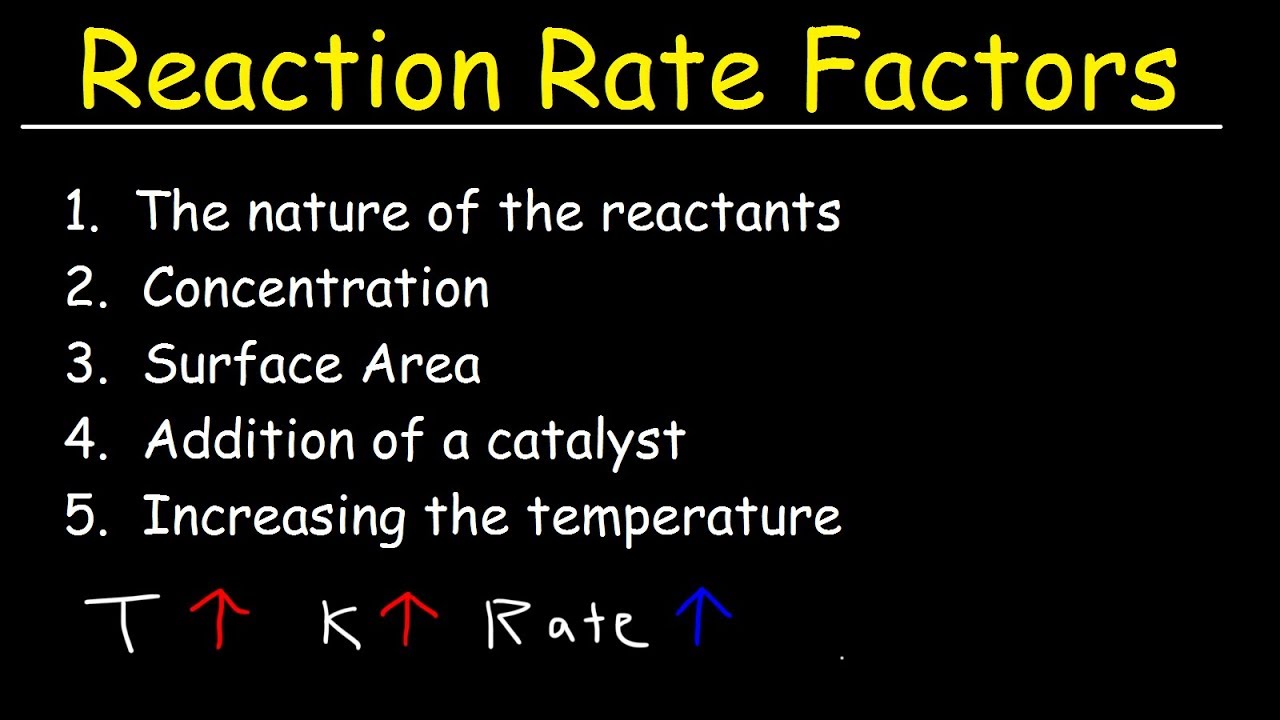
Factors Affecting The Rate Of The Reaction Chemical Kinetics YouTube
https://i.ytimg.com/vi/JpoOfrPKgmM/maxresdefault.jpg
May 25 2021 nbsp 0183 32 Chemists have identified many factors that affect the rate of a reaction The rate or speed at which a reaction occurs depends on the frequency of successful collisions Remember a successful collision occurs when two reactants collide with enough energy and with the right orientation There are four main factors that can affect the reaction rate of a chemical reaction Reactant concentration Increasing the concentration of one or more reactants will often increase the rate of reaction
Here s the best way to solve it The main factos that influence the rate of reaction RATE k A Tempe Not the question you re looking for Post any question and get expert help quickly Reactions occur when two reactant molecules effectively collide each having minimum energy and correct orientation Reactant concentration the physical state of the reactants and surface area temperature and the presence of a catalyst are
More picture related to What Four Factors Influence The Rate Of Homogeneous Reaction

Homogeneous Reaction Easy Science Reactions Ap Chemistry
https://i.pinimg.com/originals/83/f1/7a/83f17ad984b47ea2e1143b9858cbee3b.png
Leadership Factors Styles And Power
https://desklib.com/media/leadership-factors-styles-power_page_3.jpg
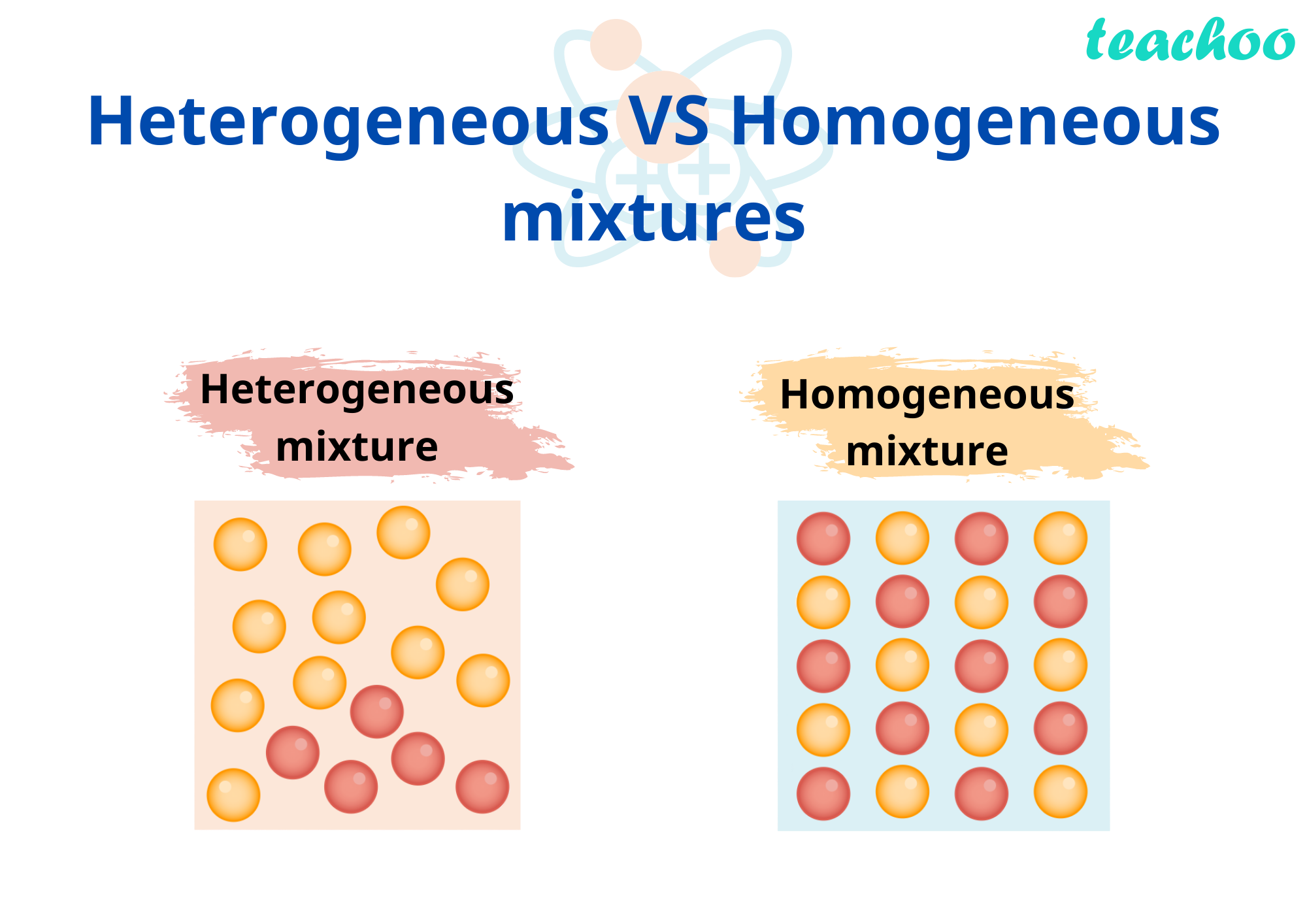
Homogeneous
https://d1avenlh0i1xmr.cloudfront.net/86b1b178-b1e1-44c7-b0cd-9c212a6e157d/homovshetero.png
There are four factors that affect the rate speed of a chemical reaction catalyst Catalysts are substances that speed up chemical reactions but can be recovered chemically unchanged at the Nov 20 2024 nbsp 0183 32 Factors that affect the rate of reaction Factors that can affect the rate of a reaction are The concentration of the reactants in solution or the pressure of reacting gases The temperature of the reaction Surface area of solid reactants The presence of a catalyst Changes in these factors directly influence the rate of a reaction
What four factors influence the rate of a homogeneous reaction 4 A reaction between the substances A and B has been found to give the following data 3A 2B 2C D A mol L B mol L Rate of appearance of C mol L hr 1 0 x 102 1 0 0 30 x 10 1 0 K 10 2 3 0 8 10 x 10 2 0 x 10 3 0 3 24 x 10 5 2 0 x 10 1 0 1 20 x 10 3 0 x 10 7 30 x 10 Using One factor affecting these different rates is that the reactions involve loss of electrons from potassium or calcium atoms and potassium has a smaller first ionization energy making loss of an electron easier

Optimizer 16 6 Windows
https://i0.wp.com/axeload.com/wp-content/uploads/2022/10/Optimizer.jpg?resize=768%2C560&ssl=1

Factors That Affect Diffusion Diagram Quizlet
https://o.quizlet.com/y8hnr1Iai4N.iZXeupAdpA_b.png
What Four Factors Influence The Rate Of Homogeneous Reaction - In many cases an increase in temperature of only 10 176 C will approximately double the rate of a reaction in a homogeneous system The rates of many reactions depend on the concentrations of the reactants Rates usually increase when the concentration of one