What Four Factors Influence The Rate Of A Reaction There are four factors that affect the rate speed of a chemical reaction catalyst Catalysts are substances that speed up chemical reactions but can be recovered chemically unchanged at the
May 25 2021 nbsp 0183 32 Describe how changing the temperature concentration of a reactant or surface area of a reaction affects the rate of a reaction Define a catalyst and how a catalyst affects the rate of a reaction According to the collision theory the rate of reaction increases with the increase in the concentration of the reactants As per the law of mass action the chemical reaction rate is directly proportional to the concentration of reactants
What Four Factors Influence The Rate Of A Reaction
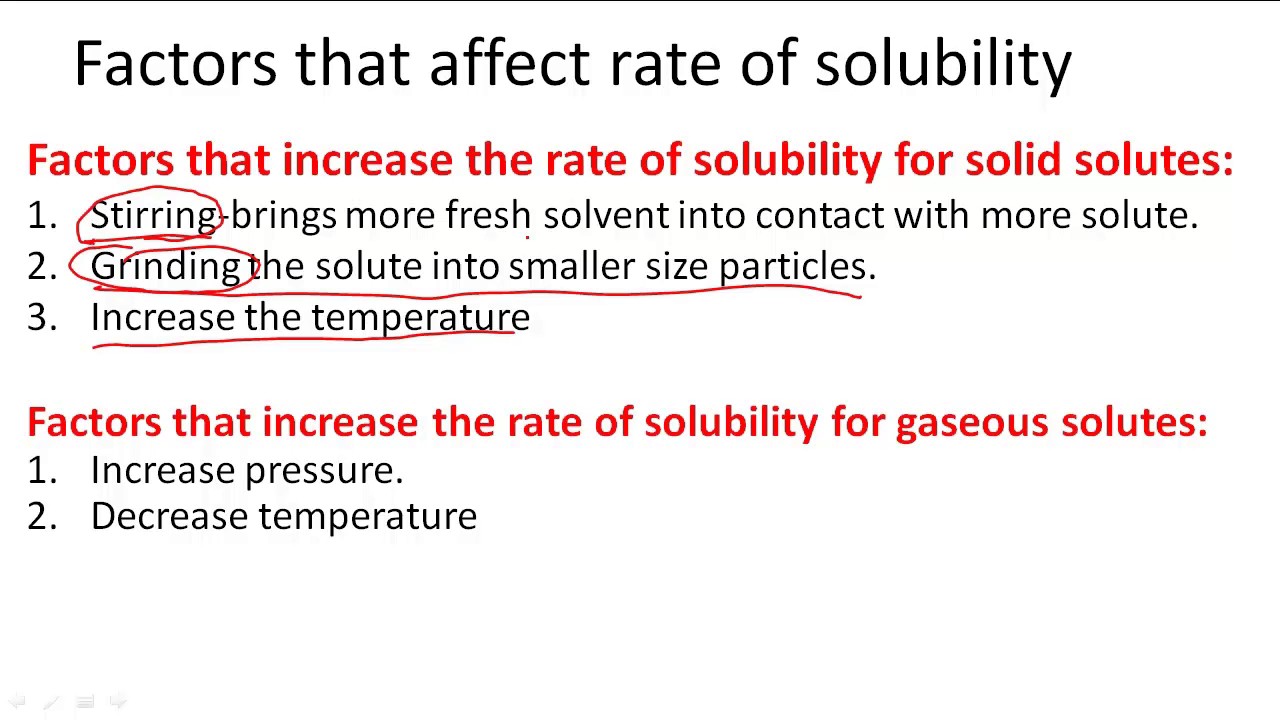
What Four Factors Influence The Rate Of A Reaction
https://i.ytimg.com/vi/pp1Sc2rPOk4/maxresdefault.jpg
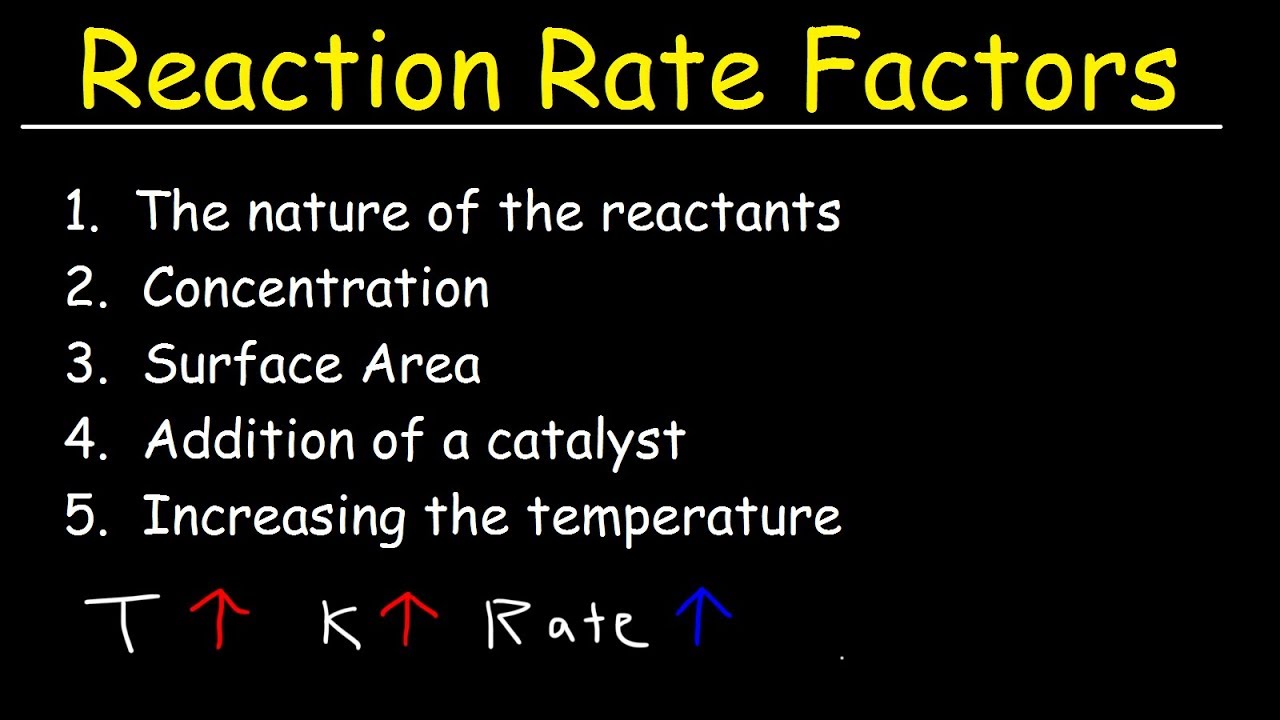
Factors Affecting The Rate Of The Reaction Chemical Kinetics YouTube
https://i.ytimg.com/vi/JpoOfrPKgmM/maxresdefault.jpg

Optimizer 16 6 Windows
https://i0.wp.com/axeload.com/wp-content/uploads/2022/10/Optimizer.jpg?resize=768%2C560&ssl=1
There are four main factors that can affect the reaction rate of a chemical reaction Reactant concentration Increasing the concentration of one or more reactants will often increase the rate of reaction Reactions occur when two reactant molecules effectively collide each having minimum energy and correct orientation Reactant concentration the physical state of the reactants and surface area temperature and the presence of a catalyst are
There are many factors that influence the reaction rates of chemical reactions include the concentration of reactants temperature the physical state of reactants and their dispersion the solvent and the presence of a catalyst Nov 20 2024 nbsp 0183 32 Factors that affect the rate of reaction Factors that can affect the rate of a reaction are The concentration of the reactants in solution or the pressure of reacting gases The temperature of the reaction Surface area of solid reactants The presence of a catalyst Changes in these factors directly influence the rate of a reaction
More picture related to What Four Factors Influence The Rate Of A Reaction

Factors That Affect Diffusion Diagram Quizlet
https://o.quizlet.com/y8hnr1Iai4N.iZXeupAdpA_b.png
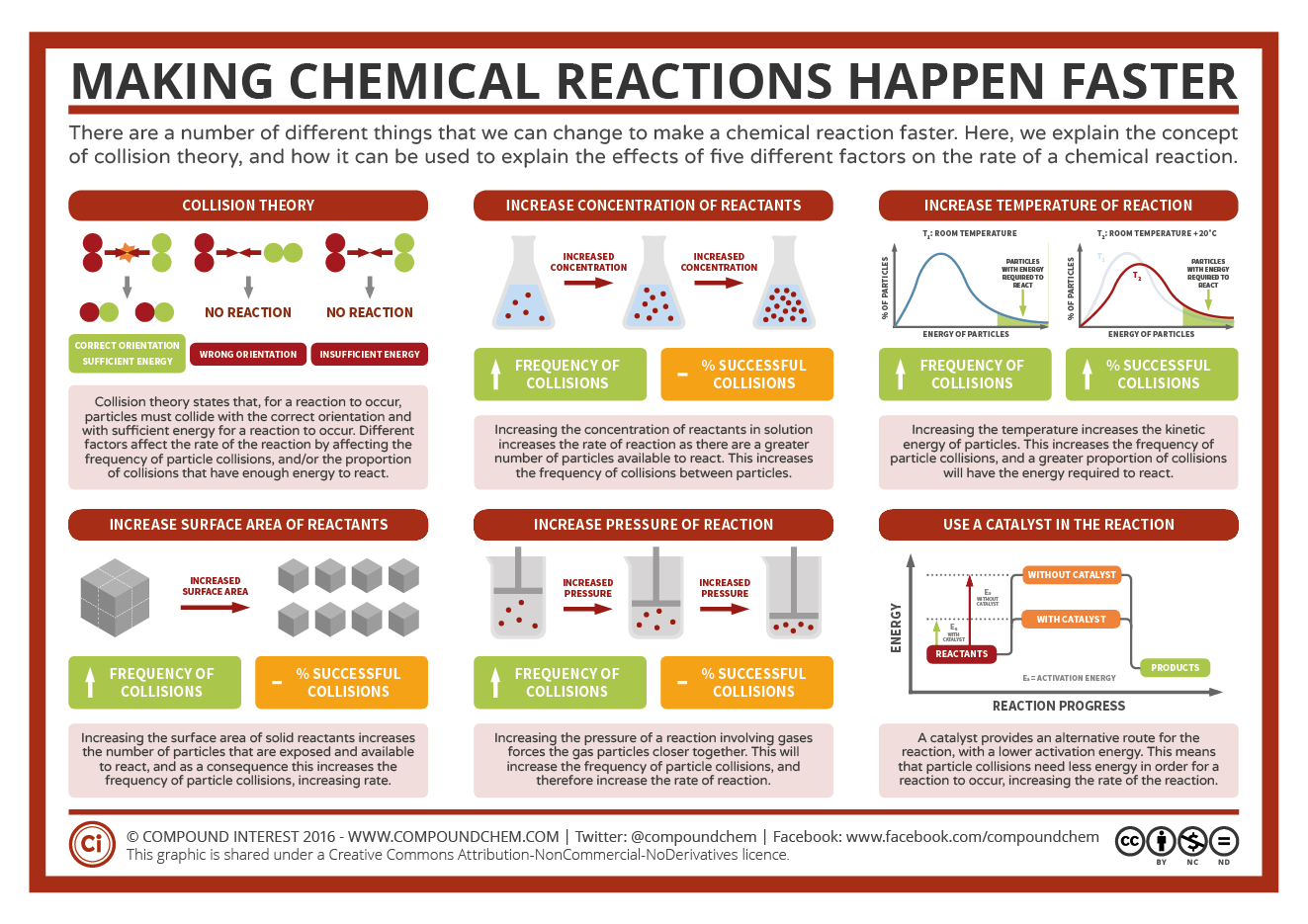
2 Bachillerato Qu mica Fisica Y Quimica EducaMadrid
http://www.compoundchem.com/wp-content/uploads/2016/02/Factors-Affecting-Rate-of-Reaction.png

Situational Factors Definition And Examples 2025
https://helpfulprofessor.com/wp-content/uploads/2023/05/situational-factors-definition-and-examples-explained-below.jpg
Nov 26 2019 nbsp 0183 32 Mixing reactants increases their ability to interact thus increasing the rate of a chemical reaction The chart below is a summary of the main factors that influence the reaction rate There is typically a maximum effect after which changing a factor will have no effect or will slow a reaction Question 3 What are the 4 factors that affect the rate of reaction Answer Concentration temperature pressure nature of reactants Question 4 The rate of chemical reaction depends on the nature of reactants because A energy required for bond breaking depends on the type and strength of bonds in reactants
Some factors that influence the speed of a chemical reaction are 1 surface area of starting reactants 2 concentration of reactants 3 temperatures The particle theory states that a solute dissolved takes place at the surface of the solvent and the larger the surface area of the particle the longer it will take to dissolve What 4 factors affect the rate of a reaction How does concentration affect the rate of reaction Most chemical reactions proceed faster if the concentration of the reactants is increased The more molecules present the more frequently they collide and the more often they react

Kinetics Collision Theory A Level ChemistryStudent
https://www.chemistrystudent.com/images/ASPhysical/kinetics/collisiontheory4.png
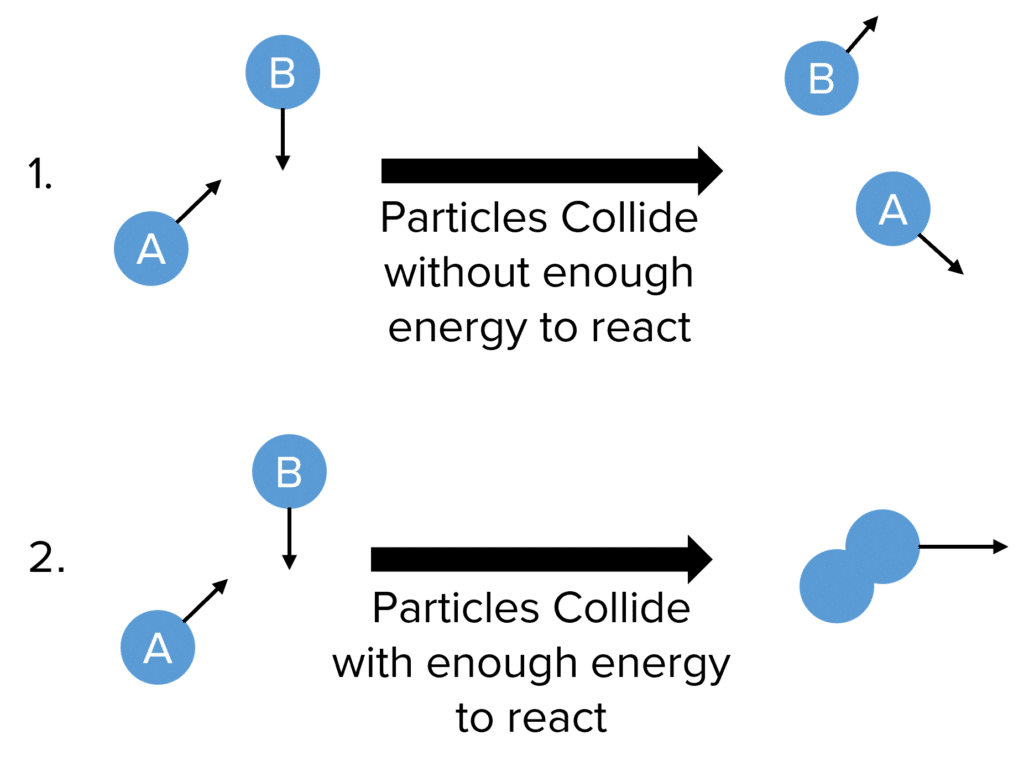
Collision Theory
https://mmerevise.co.uk/wp-content/uploads/2022/10/Collision-theory-1024x771.png
What Four Factors Influence The Rate Of A Reaction - What factors affect the rate of a chemical reaction The four main factors that affect the rate of chemical reactions are Concentration of the reactants Temperature of the reactants Surface area of the reactants Addition of a Catalyst 1 Effect of Concentration on reaction rate