What Element Results If Two Protons And Two Neutrons Are Ejected From A Radium Nucleus What element results if two protons and two neutrons are ejected from a radium nucleus To become a negative ion does an atom lose or gain an electron To become a positive ion does
What element results if two protons and two neutrons are ejected from a radium nucleus Jan 11 2023 nbsp 0183 32 When two protons and two neutrons are ejected from a radium nucleus the resulting element is radon Rn This process is known as alpha decay The atomic number
What Element Results If Two Protons And Two Neutrons Are Ejected From A Radium Nucleus

What Element Results If Two Protons And Two Neutrons Are Ejected From A Radium Nucleus
https://media.nagwa.com/404194847014/en/thumbnail_l.jpeg
/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg)
Why Protons And Neutrons Stick Together In The Nucleus
https://www.thoughtco.com/thmb/5eT1gIp6MKXhR5kmJB_qObG0RF4=/2048x1463/filters:fill(auto,1)/GettyImages-1017116892-917f9457f2bc4e4cbca2827b9d0a8966.jpg

What Would Happen To This Oxygen 16 Atom If It Loses Two Protons And
https://i.ytimg.com/vi/oEQDQTdZDaA/maxresdefault.jpg
Answer Option 3 The radium nucleus has 226 nucleons Out of 226 nucleons there are 88 protons and 138 neutrons If two protons and two neutrons are ejected from a radium To find out which element is formed when 2 protons and 2 neutrons are ejected from a radium nucleus with atomic number 88 and mass number 226 you need to consider the changes in
Because both protons and nuetrons contribute to an elements atomic mass we subtract 4 from the mass 2 protons 2 nuetrons According to the problem 2 protons were ejected so we also To determine the element that results if two protons and two neutrons are ejected from a radium nucleus we need to understand the process of radioactive decay Radium is known to undergo
More picture related to What Element Results If Two Protons And Two Neutrons Are Ejected From A Radium Nucleus
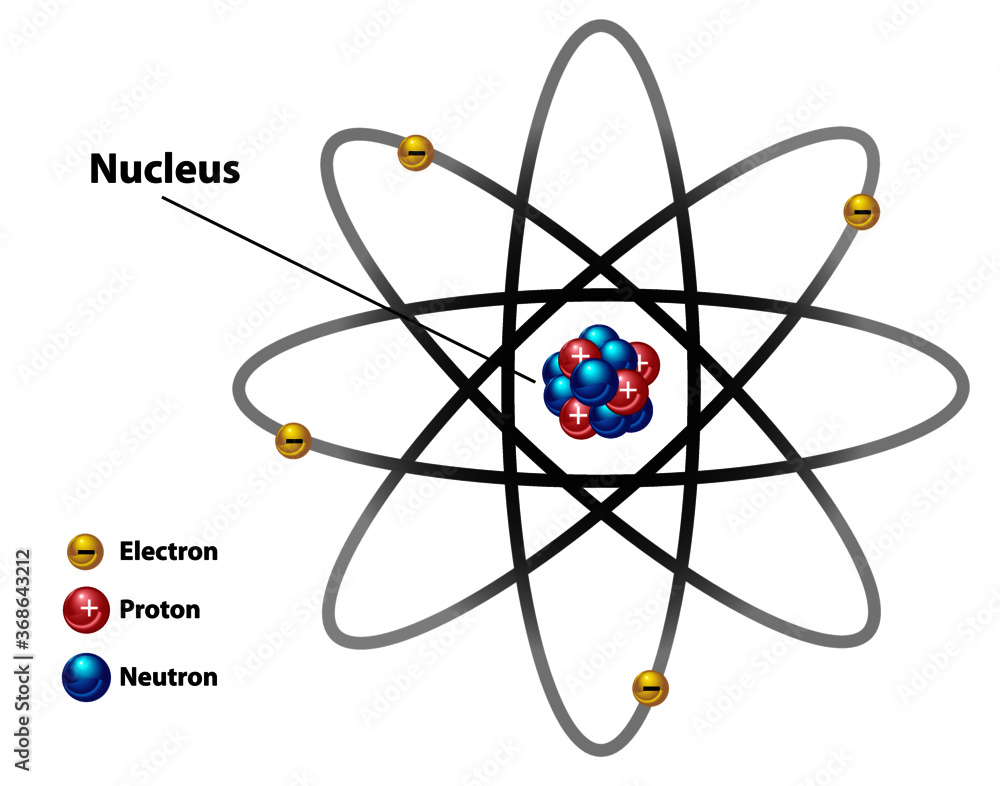
Atomic Nucleus Diagram Labeled With Electron Proton And Neutron
https://as1.ftcdn.net/v2/jpg/03/68/64/32/1000_F_368643212_ovdSJUrD6hM0crl4B03QzcQ55XlyowRS.jpg
How To Teach Finding Protons Neutrons And Electrons In An Element
https://images.squarespace-cdn.com/content/v1/61a7976ff13b3127ab967596/3011a712-f1ae-453c-afd2-f7a29d6b9f8d/2.png

Proton Neutron Electron Chart
https://physfox.s3.eu-west-2.amazonaws.com/!electricity/pne/table-pne.png
Two protons and two neutrons is an alpha particle also known as a helium nucleus 24He2 When a uranium nuclide such as 92235U ejects 24He2 the result is 92 2 235 4 X or in The resulting element when two protons and two neutrons are removed from the nucleus of oxygen b The resulting element when a pair of protons is added to the nucleus of mercury
Uranium 238 undergoes alpha decay to become thorium 234 The numbers following the chemical names refer to the number of protons plus neutrons In this reaction uranium 238 What element results if two protons and two neutrons are ejected from a radium nucleus 1 original radium 2 an isotope of radium 3 uranium 4 thorium 5 polonium 6 radon
Periodic Table Of Elements List With Protons Neutrons And Electrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d

Electron Proton Neutron
https://d1avenlh0i1xmr.cloudfront.net/9608f5f5-e406-4832-bf83-648f0c2a0609/13.-aluminium-atom-teachoo-01.png
What Element Results If Two Protons And Two Neutrons Are Ejected From A Radium Nucleus - To find out which element is formed when 2 protons and 2 neutrons are ejected from a radium nucleus with atomic number 88 and mass number 226 you need to consider the changes in