What Does Law Of Conservation Of Mass Tell Us In physics and chemistry the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter the mass of the system must remain constant over time
Law of conservation of mass states that the mass can neither be created nor destroyed but is transformed from one form to another Chemical reactions and combustion are examples of law of conservation of mass Aug 10 2024 nbsp 0183 32 Simply stated the law of conservation of mass means matter cannot be created or destroyed but it can change forms In chemistry the law is used to balance chemical equations The number and type of atoms must be the same for both reactants and products
What Does Law Of Conservation Of Mass Tell Us
What Does Law Of Conservation Of Mass Tell Us
https://cphinf.pstatic.net/mooc/20211208_91/1638936041565dis6F_JPEG/Vnm4jE4FZspTOasiLp7o.jpg
Equation Of Mass Conservation GIST
https://cphinf.pstatic.net/mooc/20211208_9/1638938359488G13vN_JPEG/Coy3ZpaFOglNuiRSoBLp.jpg

Law Of Conservation Of Mass Statement Experiment Examples and Mor
https://d1avenlh0i1xmr.cloudfront.net/0d2f027c-61a3-4a79-a216-95e1b719d23b/law-of-conservation-of-mass-teachoo-01.jpg
Dec 21 2020 nbsp 0183 32 The law of conservation of mass states that in a closed system including the whole universe mass can neither be created nor destroyed by chemical or physical changes In other words total mass is always conserved The total mass of chemicals before and after a reaction remains the same This is called the Law of Conservation of Mass
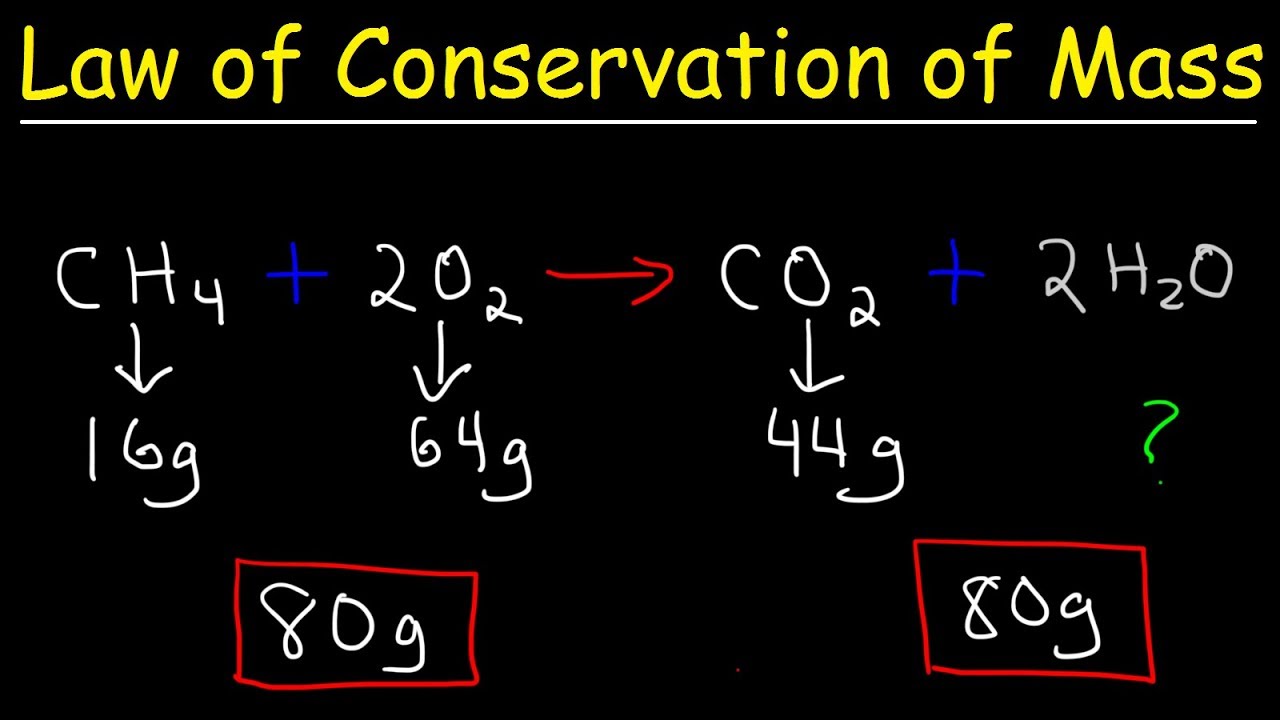
Jun 3 2024 nbsp 0183 32 According to the law of conservation of mass mass is neither created nor destroyed during a chemical reaction For example when coal is burned the carbon atom in it changes into carbon dioxide The carbon atom changes from a solid to a gas yet its mass remains constant The law of conservation of mass states that in a reaction matter can not be created or destroyed That means that the mass of all reactants in a reaction will be equal to the mass of all the products
More picture related to What Does Law Of Conservation Of Mass Tell Us

The Law Of Conservation Of Matter Exploring Its Fundamental
https://miro.medium.com/v2/resize:fit:1024/1*LuSnZ5pOYx0hTxztMrSfVA.png
Spice Of Lyfe Chemical Equation Law Of Conservation Of Mass
https://i.ytimg.com/vi/eBTNzScLUg4/maxresdefault.jpg

Simplest Explanation Law Of Conservation Of Mass YouTube
https://i.ytimg.com/vi/BjShmwXrswY/maxresdefault.jpg
Apr 18 2024 nbsp 0183 32 According to the law of conservation of mass or the law of conservation of matter matter is neither created nor destroyed in a chemical reaction or change It means the total mass of products is equal to the total mass of reactants in the chemical reaction Conservation of mass principle that the mass of an object or collection of objects never changes no matter how the constituent parts rearrange themselves Mass has been viewed in physics in two compatible ways
Jan 9 2018 nbsp 0183 32 The Law of Conservation of Mass is the principle that states that neither physical transformation nor chemical reactions create or destroy mass in an isolated system According to this principle the reactants and products in a chemical reaction must have equal masses The law of conservation of mass states that Mass can neither be created nor destroyed during the chemical combinations of matter Antoine Lavoisier who is often referred to as the father of modern chemistry performed careful experimental studies for various combustion reactions namely burning of phosphorus and mercury in the presence of air

Which Of The Following Correctly Illustrates The Conservation Of Mass
https://media.brainly.com/image/rs:fill/w:1080/q:75/plain/https://us-static.z-dn.net/files/d15/9dd1da8fe333252540cecdc70f1661e3.png
.jpg)
Law Of Conservation Of Mass Equation
https://slideplayer.com/slide/9210095/27/images/6/Law+of+Conservation+of+Mass-Example+(Figure+2.20+p148).jpg
What Does Law Of Conservation Of Mass Tell Us - The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction For example when wood burns the mass of the soot ashes and gases equals the original mass of the charcoal and the oxygen when it first reacted