What Does Gibbs Free Energy Tell Us Gibbs free energy denoted G combines enthalpy and entropy into a single value The change in free energy G is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system G can predict the direction of the chemical reaction under two conditions constant pressure
Gibbs free energy is an important value in thermodynamics that allows you to predict aspects of a chemical reaction In this tutorial we will learn why the Gibbs free energy equation is important and how to solve problems in which it is involved We will also define what a spontaneous reaction is What is specific heat Gibbs free energy also known as the Gibbs function Gibbs energy or free enthalpy is a quantity that is used to measure the maximum amount of work done in a thermodynamic system when the temperature and pressure are kept constant Gibbs free energy is denoted by the symbol G Its value is usually expressed in Joules or Kilojoules
What Does Gibbs Free Energy Tell Us

What Does Gibbs Free Energy Tell Us
https://www.chemistrylearner.com/wp-content/uploads/2022/01/Gibbs-Free-Energy-Graph.jpg

Gibbs Free Energy Definition Overview Expii
https://d20khd7ddkh5ls.cloudfront.net/screenshot_2023-04-15_at_10.45.13_pm.png
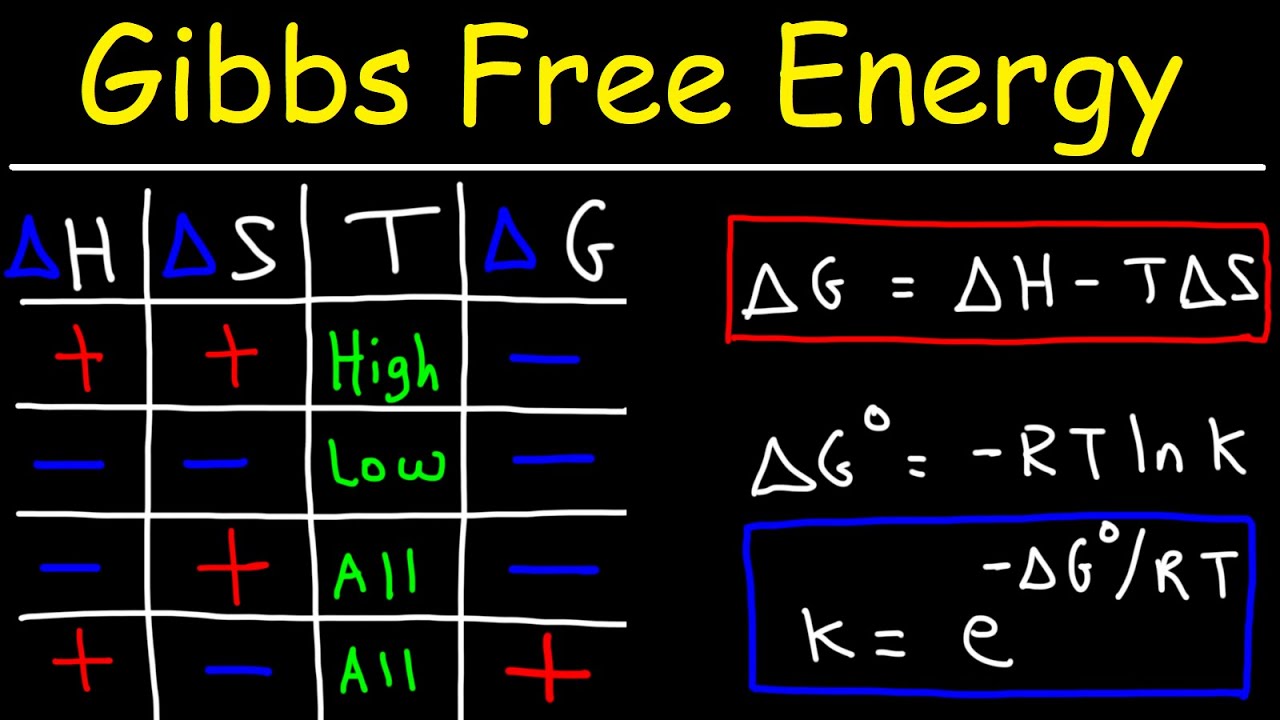
Gibbs Free Energy Entropy Enthalpy Equilibrium Constant K
https://i.ytimg.com/vi/2KuNzB0cZL4/maxresdefault.jpg
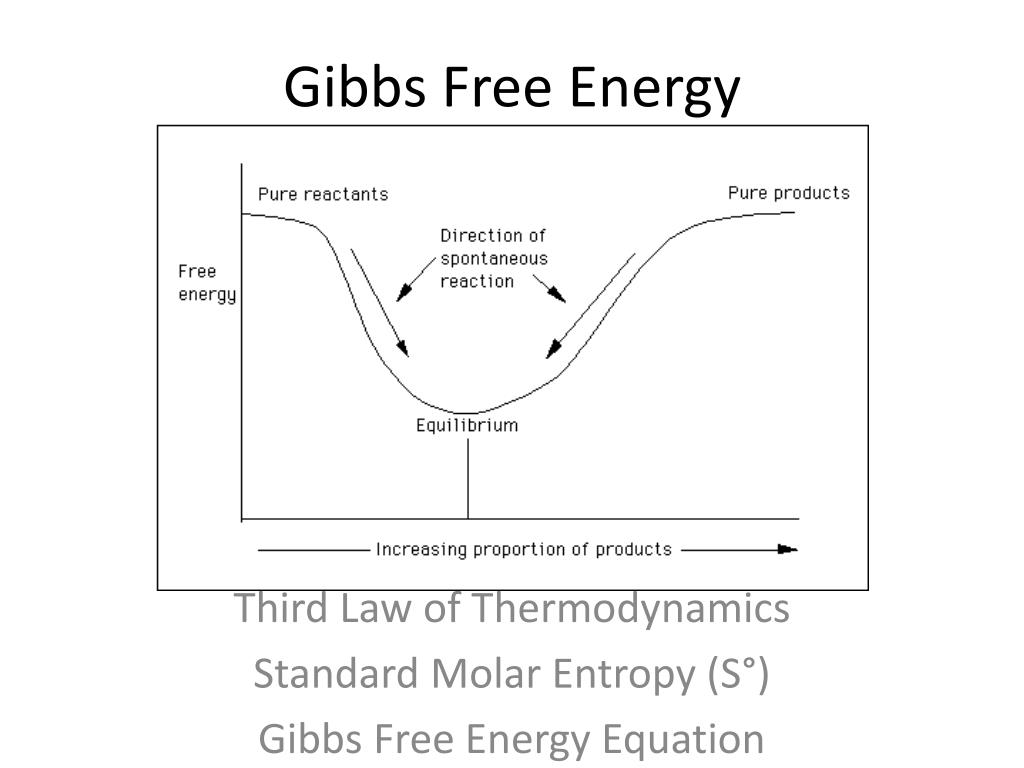
Nov 19 2024 nbsp 0183 32 In this unit we introduce a new thermodynamic function the free energy which turns out to be the single most useful criterion for predicting the direction of a chemical reaction and the composition of the system at equilibrium Apr 26 2024 nbsp 0183 32 In simple terms Gibbs free energy tells us whether a reaction will occur spontaneously If G lt 0 the reaction is spontaneous and can release energy If G gt 0 the reaction is non spontaneous and requires input of energy to occur If
American chemist J W Gibbs developed the free energy concept in the 1870s The change in Gibbs free energy is a useful term to determine the spontaneity of a thermodynamic process It is defined as the difference between the Gibbs free energies In chemical thermodynamics the Gibbs free energy gives scientists an alternative function for predicting the direction or feasibility of a reaction based on the more familiar concept of energy
More picture related to What Does Gibbs Free Energy Tell Us
Gibbs Free Energy Explained
https://image1.slideserve.com/2198185/gibbs-free-energy-l.jpg

Gibbs Free Energy How To Predict Or Calculate G And Determine If A
https://i.ytimg.com/vi/kRw6i3wwCng/maxresdefault.jpg

10 Extraordinary Facts About Gibbs Free Energy Facts
https://facts.net/wp-content/uploads/2023/09/10-extraordinary-facts-about-gibbs-free-energy-1694326789.jpg
The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system G H TS The Gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions Dec 21 2022 nbsp 0183 32 This new property is called the Gibbs free energy G or simply the free energy and it is defined in terms of a system s enthalpy and entropy as the following G H TS nonumber Free energy is a state function and at constant temperature and pressure the standard free energy change G 176 may be expressed as the following
[desc-10] [desc-11]

SOLUTION Gibbs Free Energy Ppt Studypool
https://sp-uploads.s3.amazonaws.com/uploads/services/4677156/20220824050130_6305b0aa128f6_gibbs_free_energy_pptpage2.jpg
Understanding Gibbs Free Energy
https://assets.api.gamma.app/wa7808bwsaultun/screenshots/xrld4mxee5mogas/7wknb3b6hxp7ctq/slide/3dKkvSogvb0gh8hpe7jo_NhznWE
What Does Gibbs Free Energy Tell Us - [desc-12]
