What Do You Mean By Average And Instantaneous Rate Of Reaction Average rate R t P t R t P t It occurs for a long interval of time It can be determined for multistep as well as elementary reactions Instantaneous rate is obtained when
The instantaneous rate of reaction 1 The rate of a reaction at a specified or particular point of time 2 It occurs within a short span of time 3 Instantaneous Rate of change Instantaneous Rate d x d t d P d T The average rate of a Feb 7 2019 nbsp 0183 32 The reaction rate both average and instantaneous is enabling engineers and scientists around the globe to optimize the process parameters in order to get the most desired results from a chemical reaction in the most
What Do You Mean By Average And Instantaneous Rate Of Reaction

What Do You Mean By Average And Instantaneous Rate Of Reaction
https://i.ytimg.com/vi/izCzyujhuYs/maxresdefault.jpg
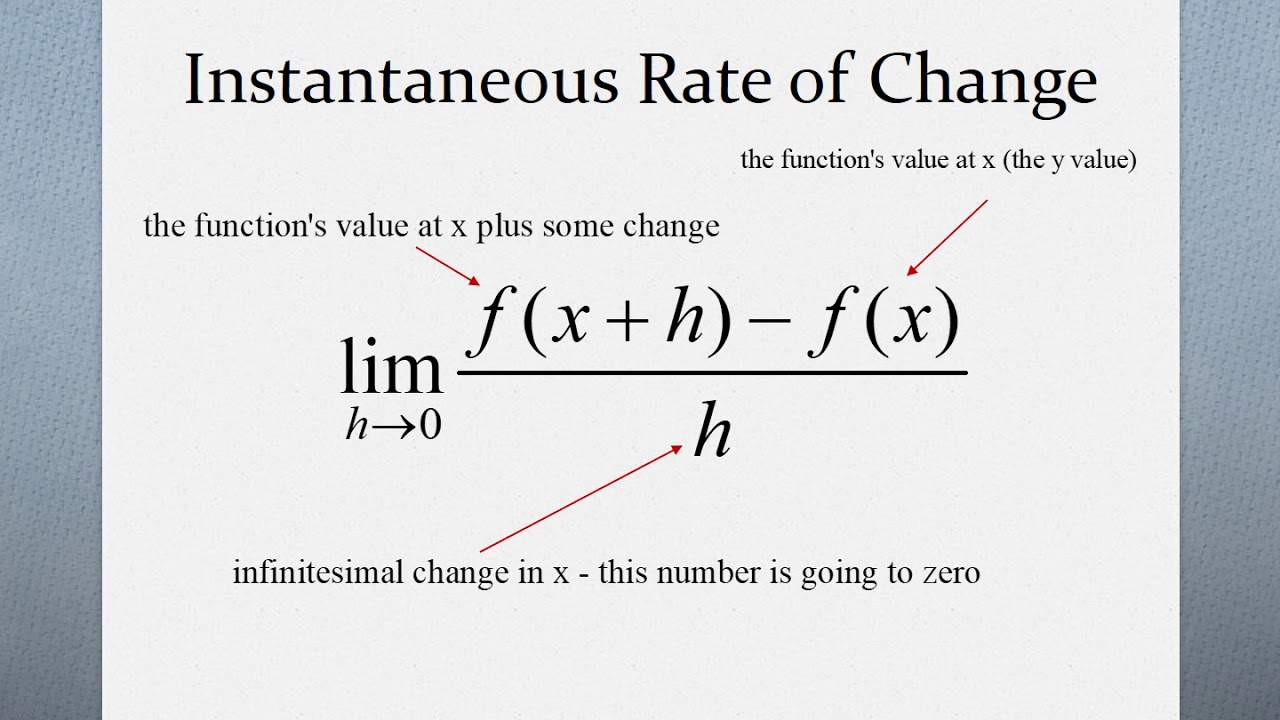
Instantaneous Rate Of Change Of A Function YouTube
https://i.ytimg.com/vi/bafE42IKhs8/maxresdefault.jpg
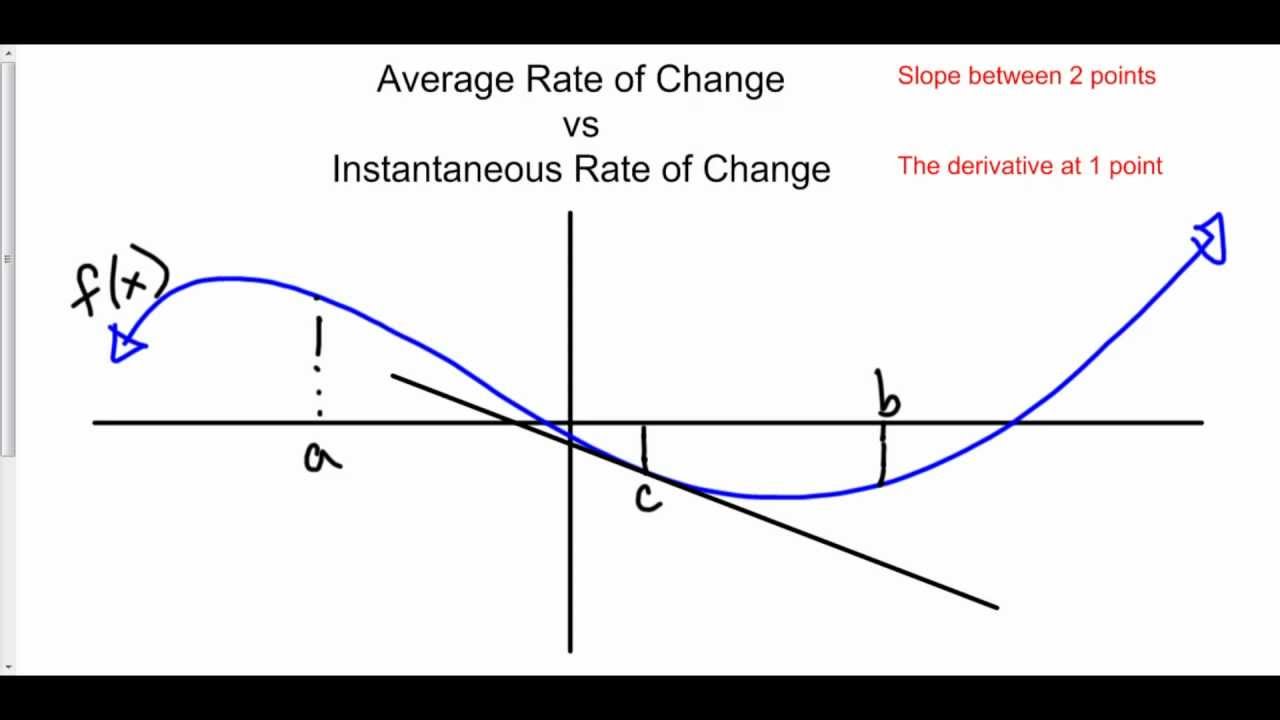
Average Vs Instantaneous Rate Of Change YouTube
https://i.ytimg.com/vi/J7VbhV_yp2U/maxresdefault.jpg
Jul 12 2023 nbsp 0183 32 Average vs Instantaneous Reaction Rates Reaction rates have the general form of change of concentration change of time There are two types of reaction rates One is called Nov 3 2018 nbsp 0183 32 It is defined as rate of change in concentration of any one of the reactant or product in a chemical reaction at particular time It is defined as the rate of reaction measured over a
Instantaneous rate of a reaction is defined as the change in the concentration in an infinitesimally small time interval Mathematical Representation of Instantaneous Rate of a Reaction Suppose the value of the term time Aug 6 2018 nbsp 0183 32 Instantaneous rate of reaction Average rate of reaction i It occurs within a short span of time It occurs during a long interval of time ii It can t be calculated for multistep
More picture related to What Do You Mean By Average And Instantaneous Rate Of Reaction

Reaction Rates How To Determine The Instantaneous Rate Of Reaction
https://i.ytimg.com/vi/Ra_enwPKw34/maxresdefault.jpg

Average And Instantaneous Rate Of Reaction Class 12 IIT JEE
https://i.ytimg.com/vi/5CCqitIYErY/maxresdefault.jpg

Chemical Kinetics 2 Average Rate Of Reaction Instantaneous Rate
https://i.ytimg.com/vi/2d8ZzHgFhzU/maxresdefault.jpg
Dec 25 2024 nbsp 0183 32 Mathematically the Average rate of reaction is given by rav R t P t The Rate of Reaction at any instant of time is the rate of change of concentration of any The instantaneous rate of reaction is the rate at some instant at a particular time Specifically it is defined as the change in concentration of the components of a reaction at an infinitely small time interval The average of the instantaneous
In this video we describe what we mean by the average rate and instantaneous rate of a chemical reaction and how we can find them from the concentration of reactant vs time graph of a Instantaneous rate of reaction It may be defined as the change in concentration of a reactant or product of a chemical reaction at a given instant So we can calculate the instantaneous
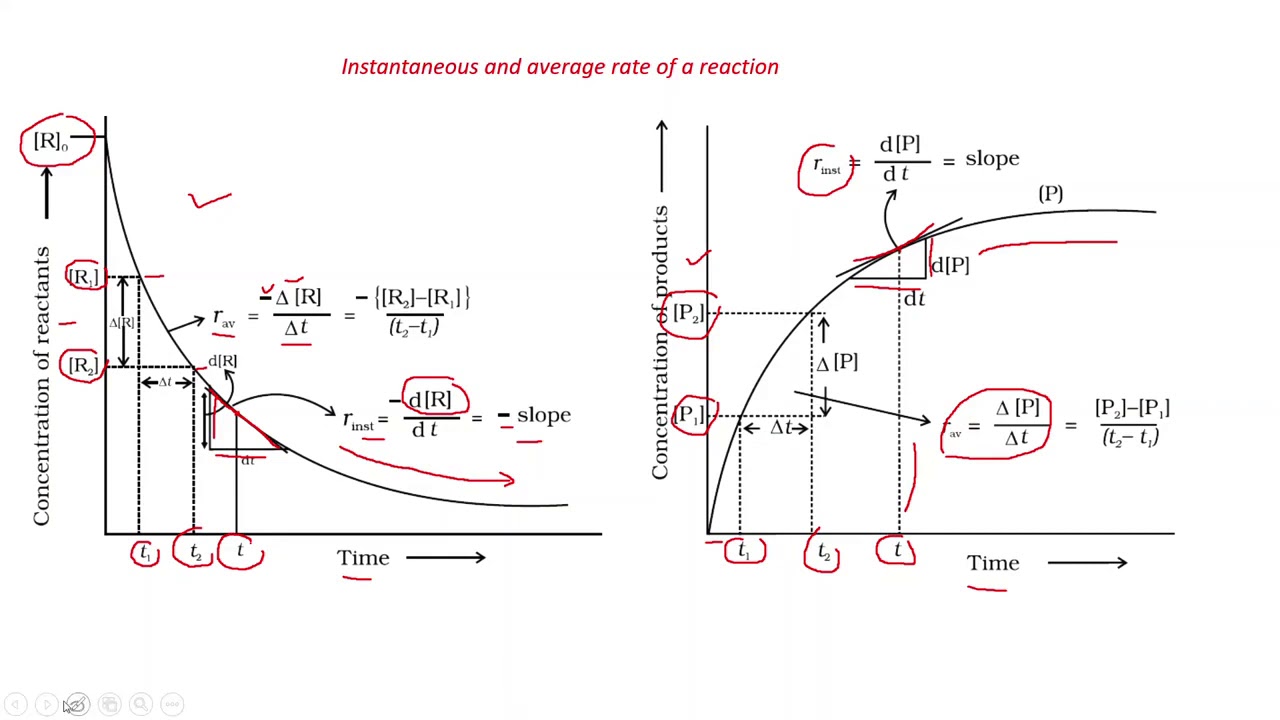
PART 1 CHEMICAL KINETICS AVERAGE AND INSTANTANEOUS RATE OF REACTIONS
https://i.ytimg.com/vi/BmdpFd0rLks/maxresdefault.jpg

Connection Between Slope Average Rate Of Change And Instantaneous Rate
https://i.ytimg.com/vi/zieu387giUo/maxresdefault.jpg
What Do You Mean By Average And Instantaneous Rate Of Reaction - The instantaneous reaction rate is the rate at a specific instant during a chemical reaction The average reaction rate corresponds to the slope of the secant that intersects the curve of a