What Can Influence Rate Of Reaction May 25 2021 nbsp 0183 32 Chemists have identified many factors that affect the rate of a reaction The rate or speed at which a reaction occurs depends on the frequency of successful collisions Remember a successful collision occurs when two reactants collide with enough energy and with the right orientation
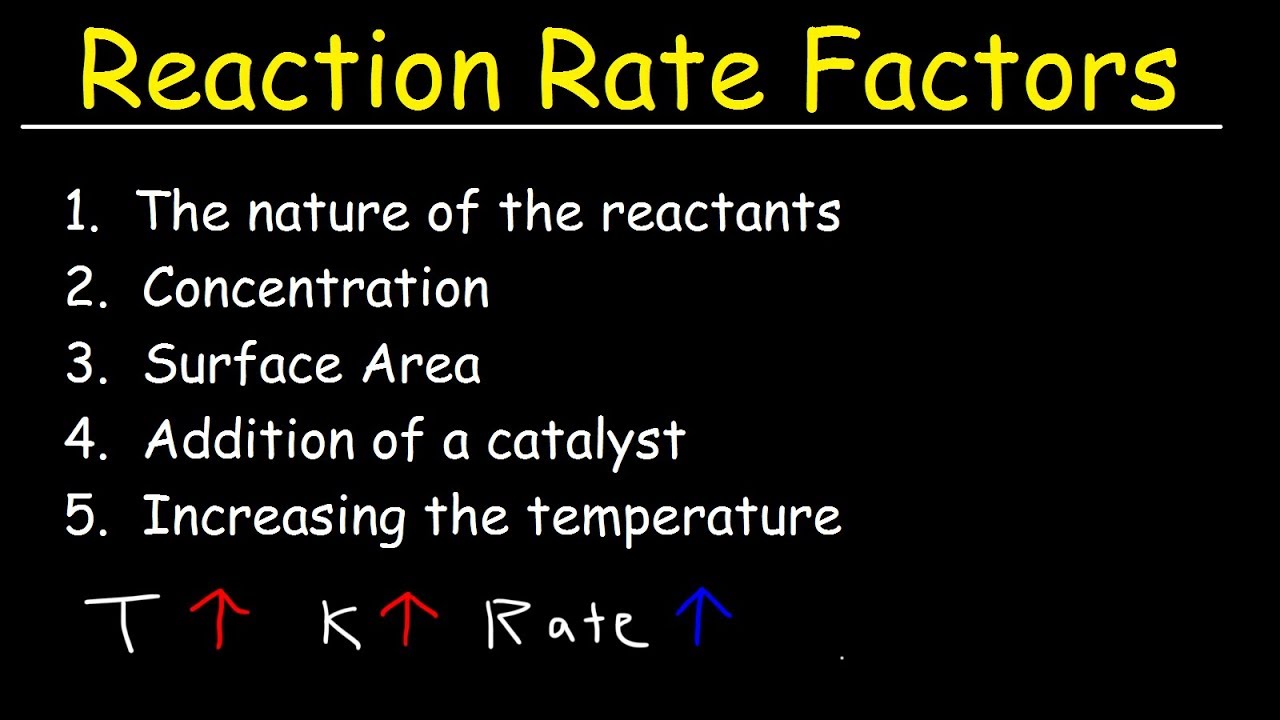
Effect of concentration on reaction rate According to the collision theory the rate of reaction increases with the increase in the concentration of the reactants As per the law of mass action the chemical reaction rate is directly proportional to the concentration of reactants There are four main factors that can affect the reaction rate of a chemical reaction Reactant concentration Increasing the concentration of one or more reactants will often increase the rate of reaction
What Can Influence Rate Of Reaction
What Can Influence Rate Of Reaction
https://i.ytimg.com/vi/JpoOfrPKgmM/maxresdefault.jpg

Chemical Kinetics 2 Average Rate Of Reaction Instantaneous Rate
https://i.ytimg.com/vi/2d8ZzHgFhzU/maxresdefault.jpg

GCSE Chemistry Factors Affecting The Rate Of Reaction 47 YouTube
https://i.ytimg.com/vi/-4HXaUBbv04/maxresdefault.jpg
There are many factors that influence the reaction rates of chemical reactions include the concentration of reactants temperature the physical state of reactants and their dispersion the solvent and the presence of a catalyst Feb 16 2020 nbsp 0183 32 The rate of reaction is affected by reactant concentration temperature pressure surface area of the particles phase effects and catalysts Chemical reactions take place when particles collide with enough energy and in the correct orientation
Nov 26 2019 nbsp 0183 32 Several factors affect the rate at which chemical reactions proceed Understanding them can help you predict the direction and speed of a chemical reaction There are four factors that affect the rate speed of a chemical reaction catalyst Catalysts are substances that speed up chemical reactions but can be recovered chemically unchanged at the
More picture related to What Can Influence Rate Of Reaction

Making Reactions Faster Factors Affecting Rates Of Reaction Gcse
https://i.pinimg.com/originals/3b/20/e5/3b20e5abe274b06f9ac7fddf018be47d.png
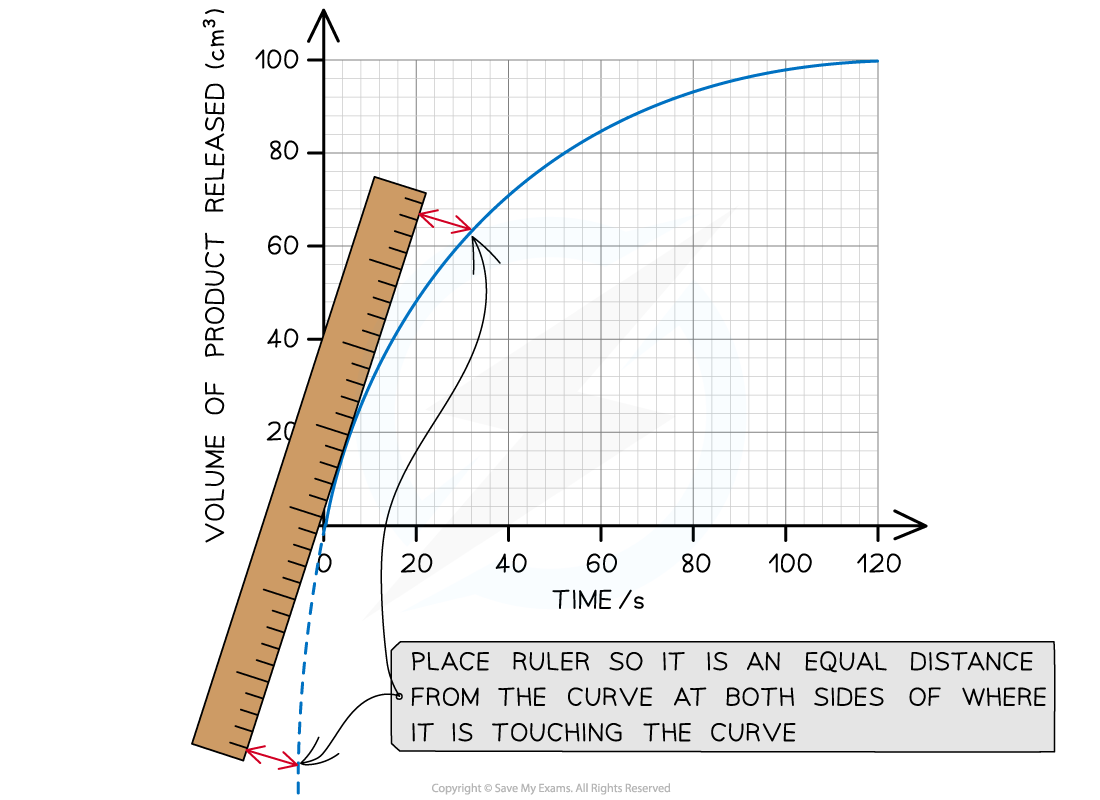
AQA A Level Biology 1 4 6 Maths Skill Using A Tangent To Find
https://oss.linstitute.net/wechatimg/2022/06/Tangent-initial-reaction-rate-3-1.png

The 5 Levels Of Influence and How To Rise To The Top
https://twobrainbusiness.com/wp-content/uploads/2023/03/5-levels-influence-business-is-good-1024x1024.jpg
Jun 19 2020 nbsp 0183 32 The rate of a chemical reaction is affected by several parameters Reactions involving two phases proceed more rapidly when there is greater surface area contact If temperature or reactant concentration is increased the rate of a given reaction generally increases as well Chemical reaction rates increase or decrease according to factors including temperature pressure and light A catalyst changes the rate of reaction but is unchanged at the end of the
Nov 20 2024 nbsp 0183 32 Factors that can affect the rate of a reaction are The concentration of the reactants in solution or the pressure of reacting gases The temperature of the reaction Learn about the the four factors that influence reaction rate temperature concentration surface area and catalysts

Factors Affecting The Rate Of Reaction Catalyst Rate Of Reaction
https://i.ytimg.com/vi/myyg4iT2uxo/maxresdefault.jpg

Examples Of Reactant
https://d20khd7ddkh5ls.cloudfront.net/concentration_affects_reaction_rate.jpeg
What Can Influence Rate Of Reaction - There are many factors that influence the reaction rates of chemical reactions include the concentration of reactants temperature the physical state of reactants and their dispersion the solvent and the presence of a catalyst