What Are Political Factors Ap Human Geography Registration of medical devices with the MHRA the UK Competent Authority does not represent any form of accreditation certification approval or endorsement by the MHRA
Please follow the Manage Registered Devices instructions to do this once your application to register the device GMDN 174 has been completed and the device is registered Device details when registering a device with the MHRA Find out more about how to register your device with the MHRA
What Are Political Factors Ap Human Geography

What Are Political Factors Ap Human Geography
https://i.ytimg.com/vi/aEnCqRmeaiI/maxresdefault.jpg

Migration Push And Pull Factors GCSE Geography YouTube
https://i.ytimg.com/vi/GG4zYsV0ME0/maxresdefault.jpg

AP Human Geo Europe CITIES Diagram Quizlet
https://o.quizlet.com/TmrtKcrx3wVszVwexQHv.w_b.jpg
Apr 16 2025 nbsp 0183 32 Step by step guide on how to register medical devices amp IVDs with the UK MHRA Includes necessary links and screen shots to walk you through the process Understanding the MHRA medical device registration process is crucial for smooth market entry In this guide we ll break down the registration process costs timelines and key requirements to help you achieve compliance effortlessly
Aug 23 2023 nbsp 0183 32 Apply to Register on the Device Online Registration System DORS A Manufacturer or UKRP must create an account on MHRA DORS before beginning to register medical devices with MHRA MHRA will email account applicants to confirm whether any account request has been accepted or rejected You must ensure all information registered with the MHRA is accurate and up to date To do this you first need to register your organisation with a manufacturer s account with the MHRA s Device Online Registration Service DORS
More picture related to What Are Political Factors Ap Human Geography

AP Human Geography YouTube
https://i.ytimg.com/vi/ex-cHIQHbo0/maxresdefault.jpg
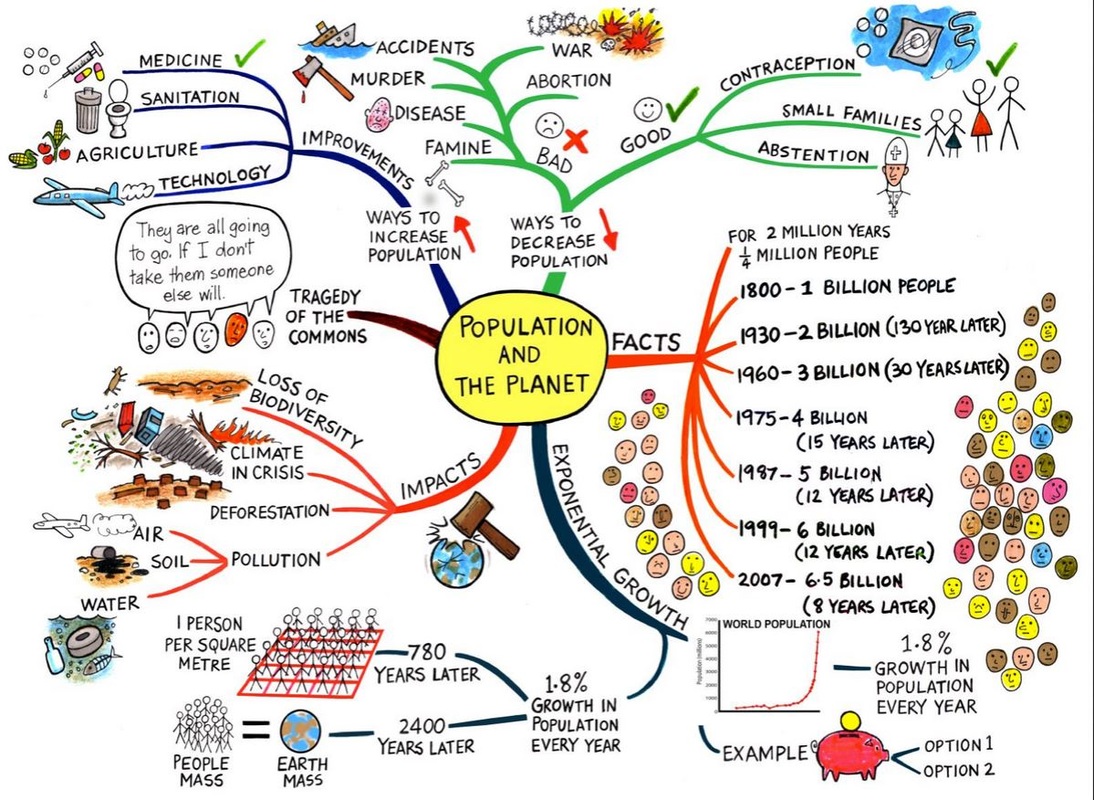
Human Geography
http://aphumangeog.weebly.com/uploads/9/6/1/1/9611587/5246695_orig.jpg
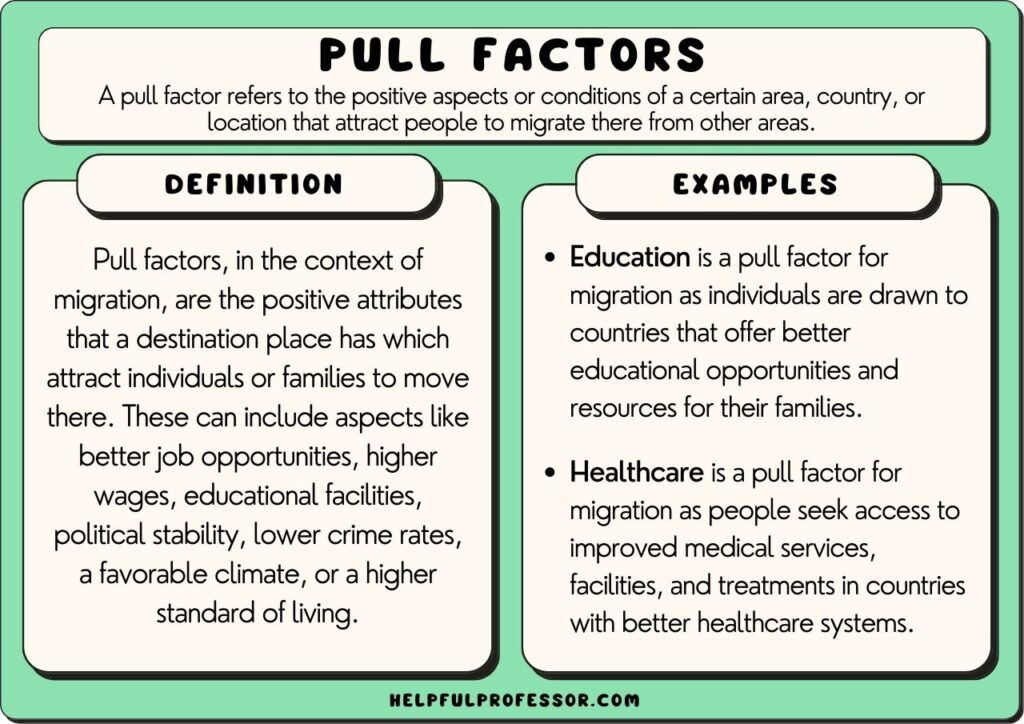
Factor Definition
https://helpfulprofessor.com/wp-content/uploads/2022/04/pull-factor-examples-and-definition-1024x724.jpg
Dec 29 2024 nbsp 0183 32 MHRA registration involves officially listing medical devices in vitro diagnostics IVDs and active implantable medical devices with the UK s regulatory authority the Medicines and Healthcare products Regulatory Agency MHRA Dec 31 2020 nbsp 0183 32 All medical devices including IVDs custom made devices and systems or procedure packs must be registered with the MHRA before being placed on the Great Britain market
[desc-10] [desc-11]

Regionalism Ap Human Geography Geogrhy Ms
https://i.ytimg.com/vi/0cHNdFn7xBM/maxresdefault.jpg

World Regions
https://m.media-amazon.com/images/I/61nmlxvUnxL.jpg
What Are Political Factors Ap Human Geography - Understanding the MHRA medical device registration process is crucial for smooth market entry In this guide we ll break down the registration process costs timelines and key requirements to help you achieve compliance effortlessly