What Are Binary Ionic Compound Naming A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion The metal part of the compound is named as the element The nonmetallic part of the compound is named by dropping the end of the element and adding ide
Sep 24 2021 nbsp 0183 32 A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion The metal cation is named first followed by the nonmetal anion as illustrated in Figure PageIndex 1 for the compound BaCl 2 Oct 12 2021 nbsp 0183 32 The rules for naming ionic compounds are a simple set of instructions that tell you how to convert a chemical formula into a written compound name Here is the list of rules along with examples of name of binary and polyatomic compounds
What Are Binary Ionic Compound Naming

What Are Binary Ionic Compound Naming
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/fa7fa0ad3fb5ff1b07f44e6ce770a81f/thumb_1200_1562.png
How To Name Ionic Compounds With Transition Metals YouTube
https://i.ytimg.com/vi/eM5mDnQX0k8/maxresdefault.jpg

Worksheet Naming Ionic Compounds
https://www.housview.com/wp-content/uploads/2018/05/collection_of_ionic_compounds_naming_worksheet_8.jpg
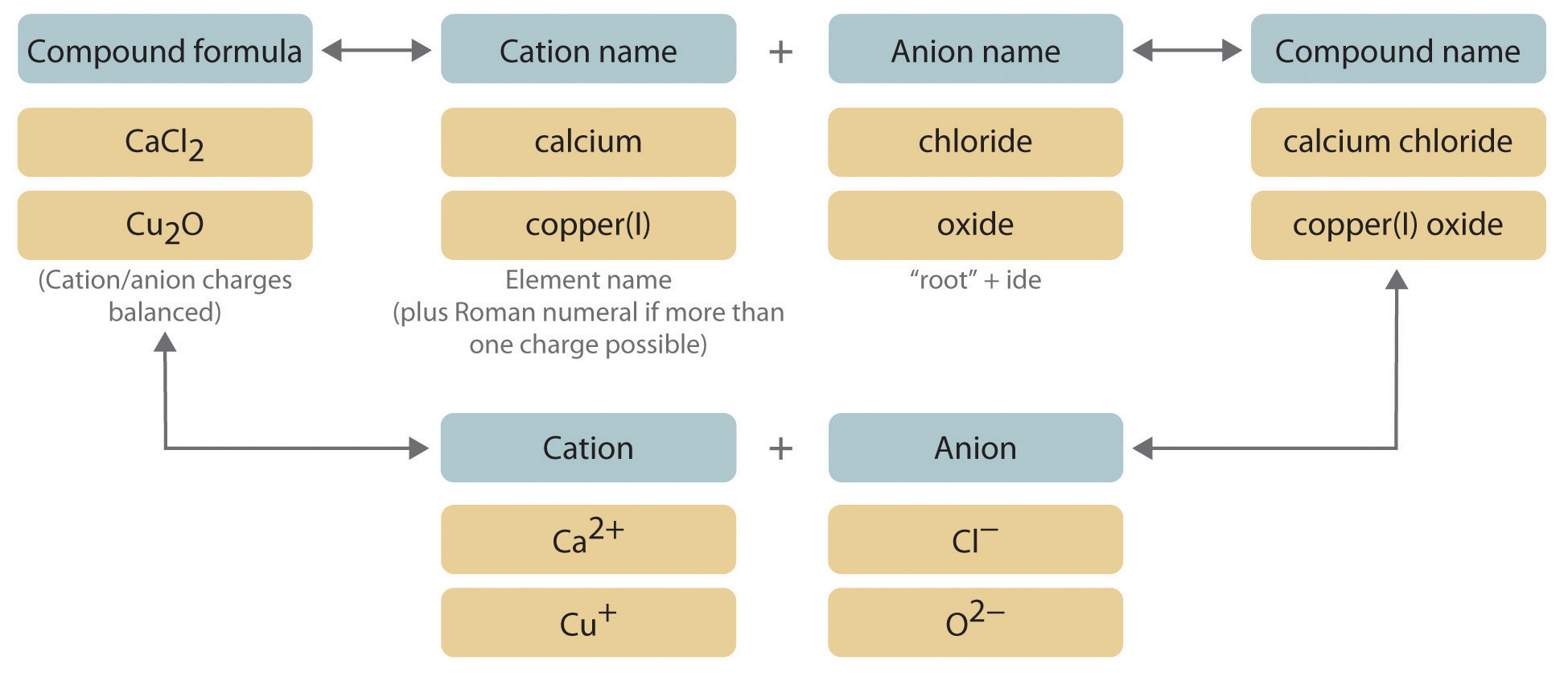
There are several rules for naming binary ionic compounds First name the metal atom followed by the nonmetal with an appropritate suffix Naming Binary Ionic Compounds Naming ionic compounds is simple combine the name of the cation and the name of the anion in both cases omitting the word ion Do not use numerical prefixes if there is more than one ion necessary to balance the charges NaCl is sodium chloride a combination of the name of the cation sodium and the anion
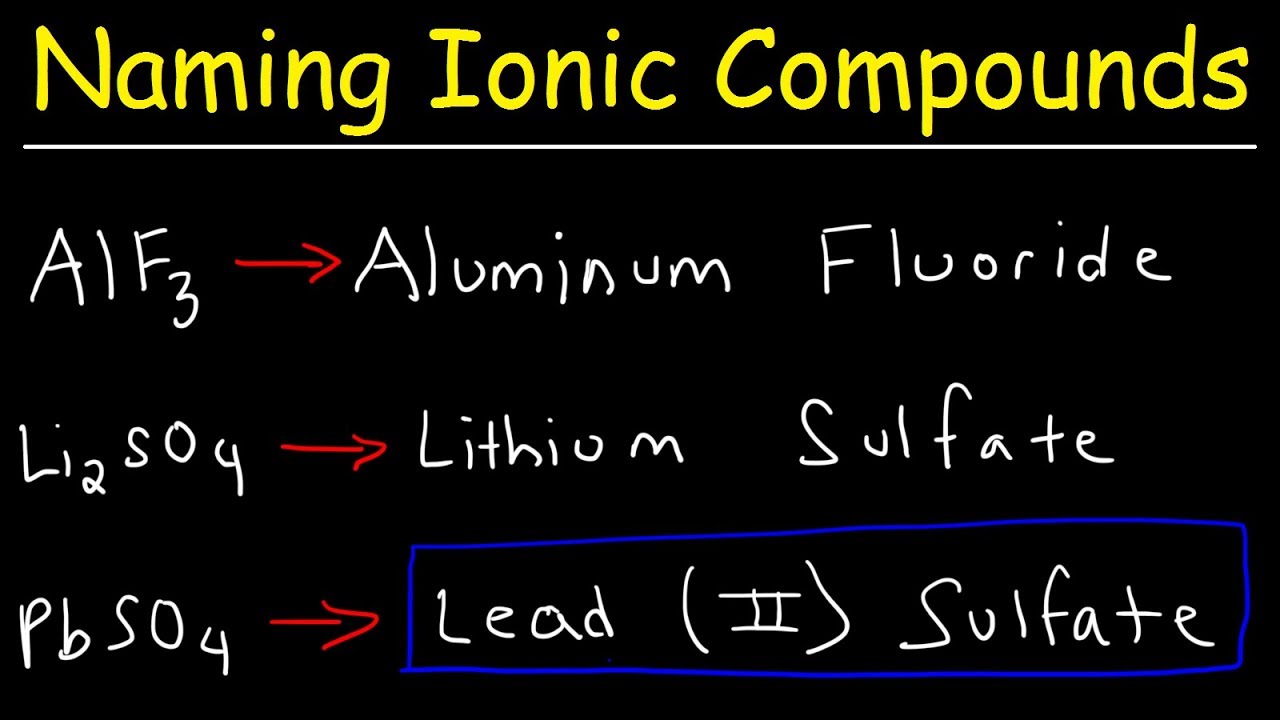
To name a binary ionic compound identify the ions and list the cation first followed by the anion Remember group 1 and 2 cations inherit the name of the metal they are derived from and monoatomic anions take the ending ide For example K 2 S Potassium sulf ide CaBr 2 Calcium brom ide AlCl 3 Aluminum chlor ide MgO Magnesium ox ide 1 The Cation positive ion is named first the Anion second 2 Monoatomic Cations take the element name Na gt Sodium Ca 2 gt Calcium 3 Monoatomic Anions take the elements name and ends with quot ide quot Cl gt Chloride NaCl gt Sodium Chloride Li 3 N gt Lithium Nitride
More picture related to What Are Binary Ionic Compound Naming
Naming Ionic Compounds
https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/section_06/31092c38512b1a22518b627df41aca40.jpg

Ionic Compounds Naming
https://s3.studylib.net/store/data/009291540_1-c9896bcec5ca814c3605073966d45410.png

Naming Compounds
https://i.ytimg.com/vi/dzjkBan26tQ/maxresdefault.jpg
Apr 3 2021 nbsp 0183 32 Binary Ionic Compounds The first atom or cation in a binary ionic compound is a metal while the second atom or anion is a nonmetal Binary ionic compounds tend to have relatively high melting and boiling points due to the ionic bond They often dissolve in water to yield electrolytes Binary ionic compounds As the name suggests these are formed from two different elements a metal and a non metal There may also be more than one of each element in the compound It s not to be confused with a diatomic compound which has multiple atoms of the same element
What is a Binary Ionic Compound A binary ionic compound is an ionic compound that contains only two elements One of the elements is a metal and serves as the cation The other element is a nonmetal and serves as the anion Here are a few examples of binary ionic compounds written in formula form How to Name Binary Acids and Oxyacids in General Chemistry Acids are a type of covalent compound that release hydrogen ions when dissolved in water Binary acids are composed of hydrogen and one other element while oxyacids contain hydrogen oxygen and another element
Naming Ionic Compounds SliderBase Worksheet Template Tips And Reviews
http://www.sliderbase.com/images/referats/141b/(28).PNG
Formulas And Nomenclature Answers
http://1.bp.blogspot.com/_HSyZWoBHdDg/TMsPAJkvAvI/AAAAAAAAAlE/IkQIJRXDN2k/s1600/Naming+Ionic+Compounds+WS.JPG
What Are Binary Ionic Compound Naming - Learn how to name binary ionic compounds and see examples that walk through sample problems step by step for you to improve your chemistry knowledge and skills
.PNG)