Trends Of The Periodic Table Worksheet Part 2 Answer Key EXTRA PRACTICE Periodic Trends Practice 2 WS ANSWER KEY copy Extra Practice Periodic Trends 1 ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius a Li C b Li Na K 165 L k e Al Cl Ga C I 2
In this activity you will look at a few periodic trends that can help you make those predictions Like most trends they are not perfect but useful just the same 1 Consider the data in Model 1 on the following page a Each element has three numbers listed under it Which value represents the atomic radius b Directions Use your notes to answer the following questions 1 Rank the following elements by increasing atomic radius carbon aluminum oxygen potassium 2 Rank the following elements by increasing electronegativity sulfur oxygen neon aluminum 3 Why does fluorine have a higher ionization energy than iodine compared to Iodine
Trends Of The Periodic Table Worksheet Part 2 Answer Key

Trends Of The Periodic Table Worksheet Part 2 Answer Key
https://sciencenotes.org/wp-content/uploads/2022/09/Periodic-Table-Questions-and-Answers-1024x683.png
13 Periodic Table Worksheet Fill In Worksheeto
https://www.worksheeto.com/postpic/2012/03/periodic-table-worksheets_574440.png
Blank Periodic Table Worksheet
http://www.worksheeto.com/postpic/2015/08/periodic-table-trends-worksheet_180933.png
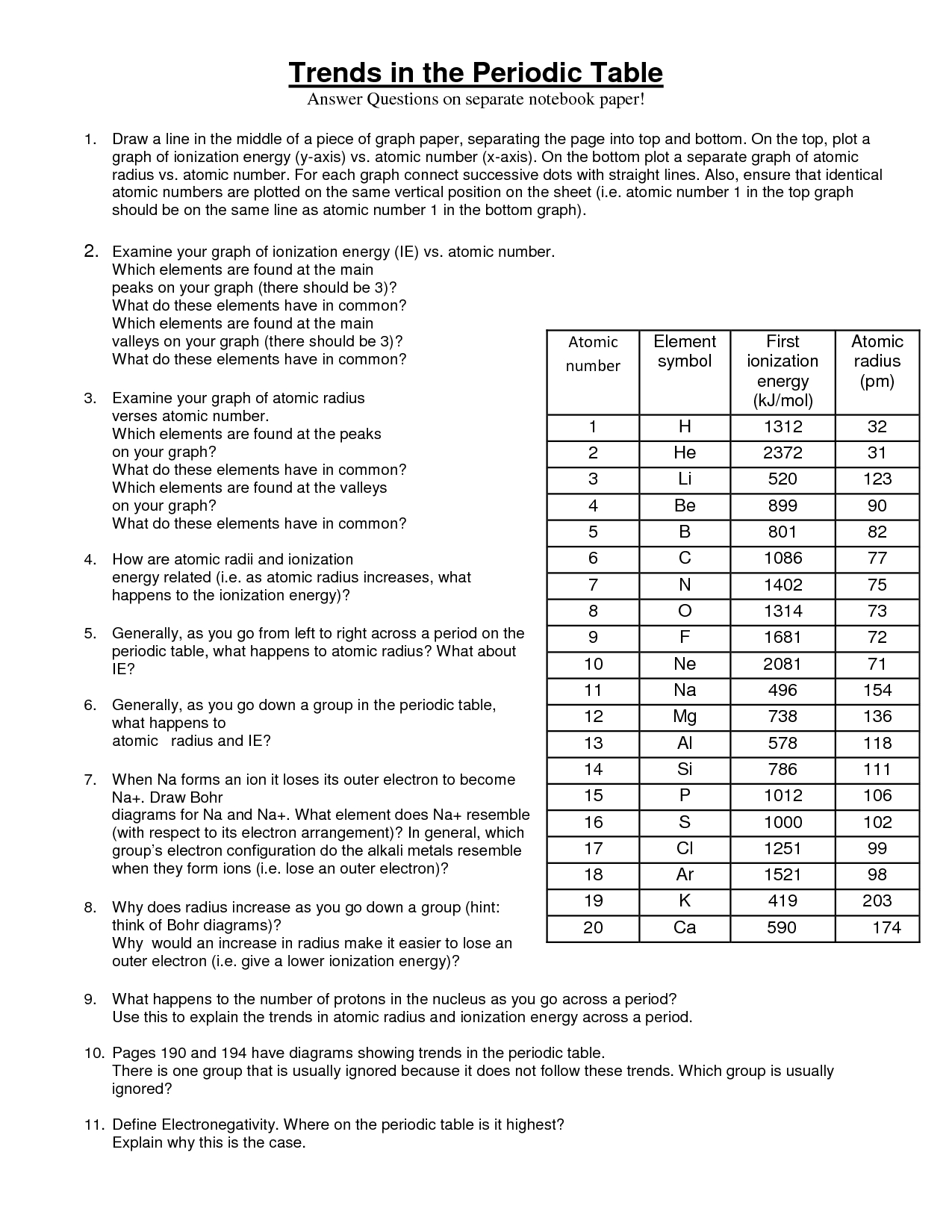
Explore Examine other groups and periods in the periodic table to see if the same trends exist What trends do you see in ionization energy down a group and across a period Ionization energy tends to decrease down a group and increase across a period Explore Examine other groups and periods in the periodic table to see if the same trends exist What trends do you see in ionization energy down a group and across a period Ionization energy tends to decrease down a group and increase across a period
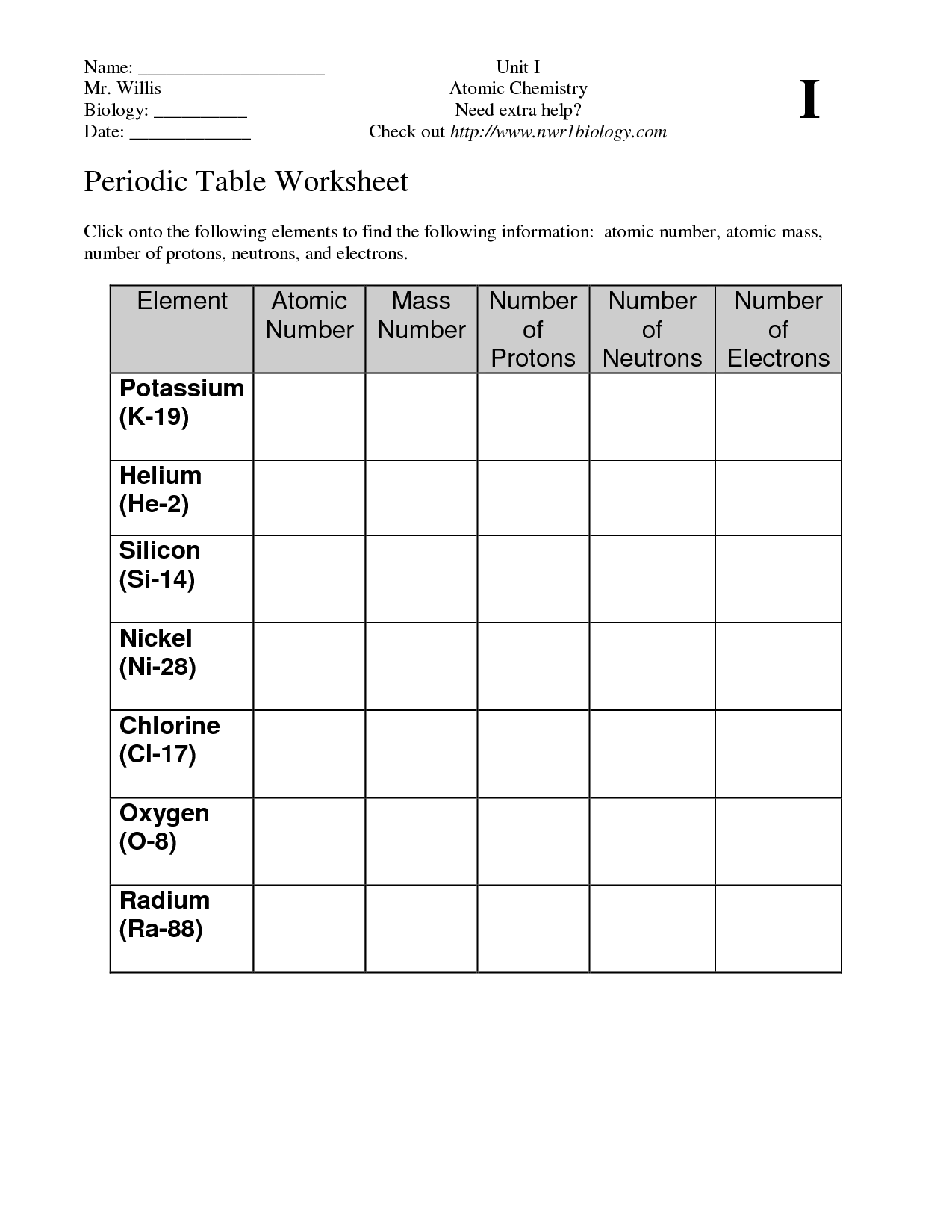
3 What trends do you notice for the atomic radii of Period 3 4 Explain why this trend occurs 5 Ionization energy is the amount of energy required to remove an electron from an element Using the ionization energies of the elements in Period 2 listed below make a line graph the values vs atomic number Period 2 It provides the answers to questions about classifying elements as metals nonmetals or metalloids It also answers questions about trends in atomic radius ionization energy and electronegativity across the periodic table including ordering elements by these properties and defining each term
More picture related to Trends Of The Periodic Table Worksheet Part 2 Answer Key
8 Images Exploring Trends Of The Periodic Table Worksheet Answer Key
https://alquilercastilloshinchables.info/wp-content/uploads/2020/06/Periodic-Table-Trends-Worksheet-Answer-Key-Periodic-table-....png

Periodic Table Worksheet 1 Answer Key Uncategorized Resume Examples
https://i2.wp.com/thesecularparent.com/wp-content/uploads/2020/02/chemistry-periodic-table-worksheet-answer-key.jpg

Periodic Table Worksheets With Answers Worksheets
https://i.pinimg.com/originals/f7/9c/7b/f79c7b6beb6a908147847c68042d90b0.jpg
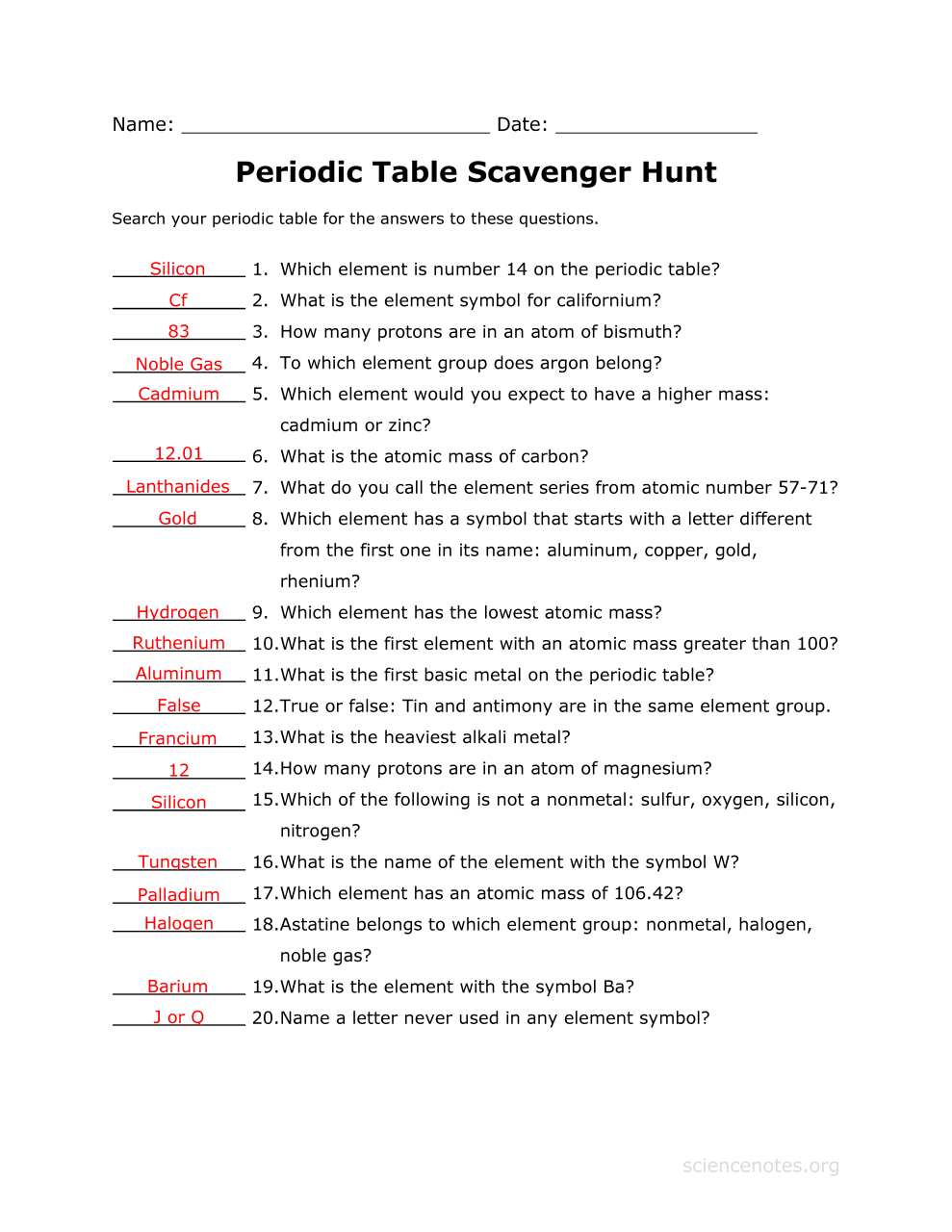
The Periodic Table Part 2 Answer Key ThePeriodicTablePart2AnserKey Back to Periodic Table Part 2 Worksheet Back to Worksheets Back to The Periodic Table and its Design Study Guide Leave a Reply Cancel reply Your email address will not be published Periodic Trends and Ionic Compounds 8 Covalent Bonds and Lewis Dot Structures Nov 27 2023 nbsp 0183 32 Fill in the best answer for each of the following a A vertical column on the periodic table is called a group b The periodic table was first arranged by the scientist Mendeleev c The elements in the periodic table are presently arranged in order of increasing atomic number
Periodic Trends The answer key discusses various periodic trends such as atomic radius electronegativity ionization energy and electron affinity It explains how these trends change across the periodic table and the factors that influence them Title Microsoft Word 4 14a Periodic Table Trends Wkst Key doc Author Brent White Created Date 7 6 2005 8 27 55 PM

Periodic Table Of Elements poster educational Print Instant Etsy UK
https://i.pinimg.com/originals/3f/87/81/3f87817e6a545f78b8ccb6f14144c68e.jpg
Answer Key Periodic Table Trends Worksheet Thekidsworksheet
https://thekidsworksheet.com/wp-content/uploads/2022/01/769c6e491dd317426d73c2cb954001da-7.png
Trends Of The Periodic Table Worksheet Part 2 Answer Key - These patterns are referred to as periodic trends Question How do atomic radius ionization energy and electron affinity change throughout the periodic table 1 Predict Based on your investigations in activities A and B predict where in the periodic table you will typically find the following Largest atoms smallest atoms highest ionization