The Element Lithium Has 3 Protons And 4 Neutrons Lithium is the 3rd element in the periodic table and has a symbol of Li and atomic number of 3 It has an atomic weight of 6 940 and a mass number of 7 Lithium has three protons and four
Dec 8 2020 nbsp 0183 32 Lithium is a chemical element with atomic number 3 which means there are 3 protons in its nucleus Total number of protons in the nucleus is called the atomic number of Jun 30 2023 nbsp 0183 32 Lithium is an alkali metal with the atomic number 3 and an atomic mass of 6 941 g mol This means that lithium has 3 protons 3 electrons and 4 neutrons 6 941 3 4
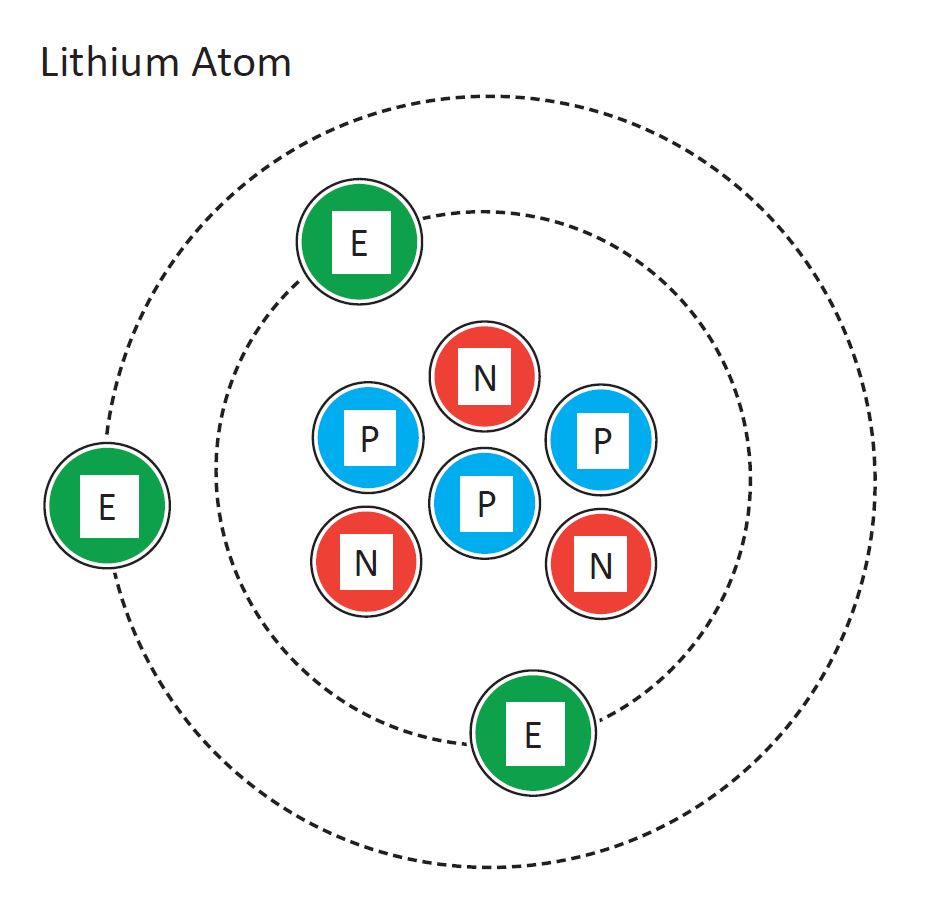
The Element Lithium Has 3 Protons And 4 Neutrons
The Element Lithium Has 3 Protons And 4 Neutrons
https://dqo7qtr60k265.cloudfront.net/ImageshareResourceFiles/Science+Concepts/Atom%2C+Lithium/Atom%2C+Lithium.JPG
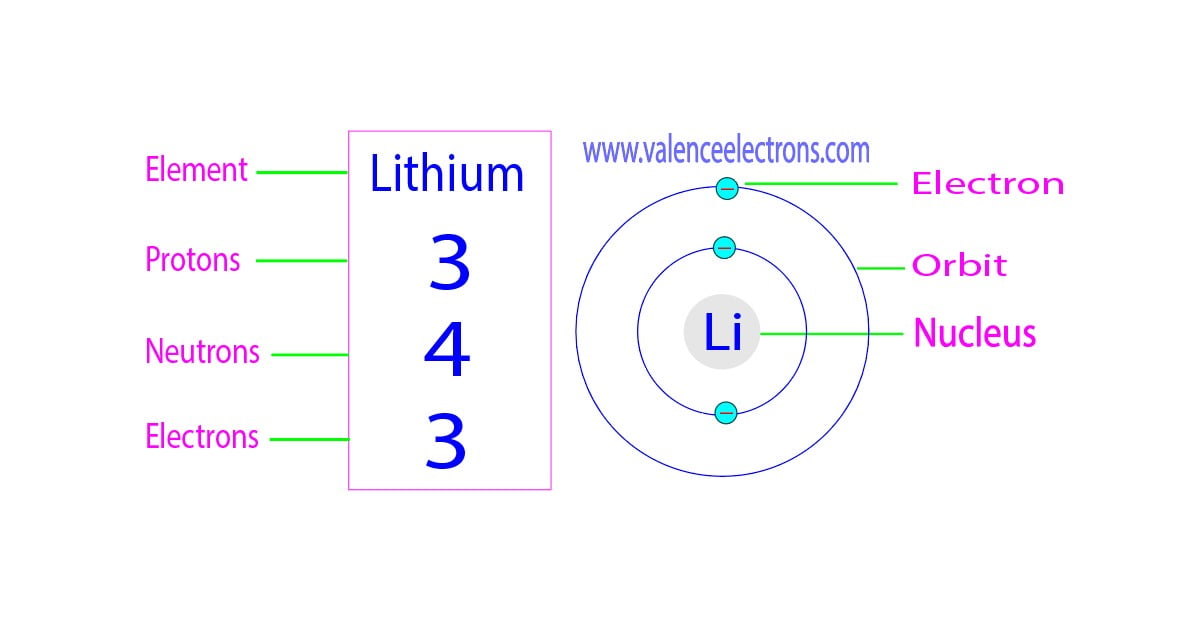
How Many Protons Neutrons And Electrons Does Lithium Have
https://valenceelectrons.com/wp-content/uploads/2022/06/Lithium-protons-neutrons-electrons.jpg

Which Element Has 9 Protons YouTube
https://i.ytimg.com/vi/65aBP4Wo07Q/maxresdefault.jpg
Aug 6 2024 nbsp 0183 32 The element that has properties most similar to lithium is the one with 4 protons 4 neutrons and 3 electrons This is because it is only one proton away from lithium s 3 protons Lithium is the 3rd element of the periodic table so its atomic number is 3 Therefore a lithium atom has three protons four neutrons and three electrons
May 25 2023 nbsp 0183 32 Lithium has 3 protons 4 neutrons and 3 electrons But how will you find the number of protons neutrons and electrons in Lithium Li Well it is very easy to find the protons neutrons and electrons of lithium atom The number of protons and number of electrons in Lithium atom are 3 and number of neutrons are 4 The model has three protons blue and four neutrons gray in the nucleus with three electrons red moving around the nucleus
More picture related to The Element Lithium Has 3 Protons And 4 Neutrons

Periodic Table Element Proton Neutron Electron Periodic Table Printable
https://i0.wp.com/www.periodictableprintable.com/wp-content/uploads/2022/07/periodic-table-of-elements-list-with-protons-neutrons-and-electrons-scaled.jpg?resize=1536%2C1164&ssl=1
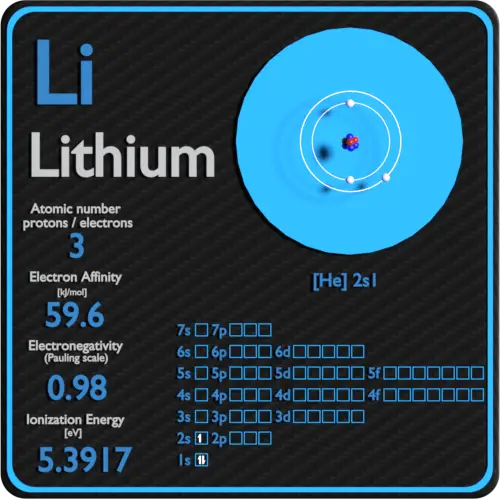
Lithium Periodic Table And Atomic Properties
https://material-properties.org/wp-content/uploads/2020/10/Lithium-affinity-electronegativity-ionization.png

Lithium Atom Structure
https://i.ytimg.com/vi/1BKXu5gk6oA/maxresdefault.jpg
Jan 7 2025 nbsp 0183 32 The atomic mass of lithium is 6 941 so we ll take the roundup value as 7 And the atomic number of lithium is 3 Subtract the atomic number 3 from the atomic mass 7 Hence lithium has a total of 7 3 4 neutrons May 21 2024 nbsp 0183 32 No lithium has 3 protons 3 electrons and usually either 3 or 4 neutrons The most common isotope of lithium has 4 neutrons but there is a less common isotope with 3
A 2 dimensional representation of the Bohr Model of a Lithium 7 isotope with 3 protons and 4 neutrons in the nucleus and 2 electrons in the first shell and 1 in the outer shell Lithium is a Group 1 element on the Periodic Table with 3 protons Mar 2 2021 nbsp 0183 32 For example a lithium atom Z 3 A 7 amu contains three protons found from Z three electrons as the number of protons is equal to the number of electrons in an atom and
Periodic Table Of Elements List With Protons Neutrons And Electrons
https://lh6.googleusercontent.com/proxy/cHmJ4HVruNArIS-g6hNX89WoA7T5gTJJW20eAPyow--172JAhLyOQCyxKdJr3UfUzF_joaggZotDXoiZhW_KT36u-nz_BwYXA2Ja7QWs2wxcbBSZT9x-3qxMsVQkRRJbVs5MypfI8nI84w=s0-d
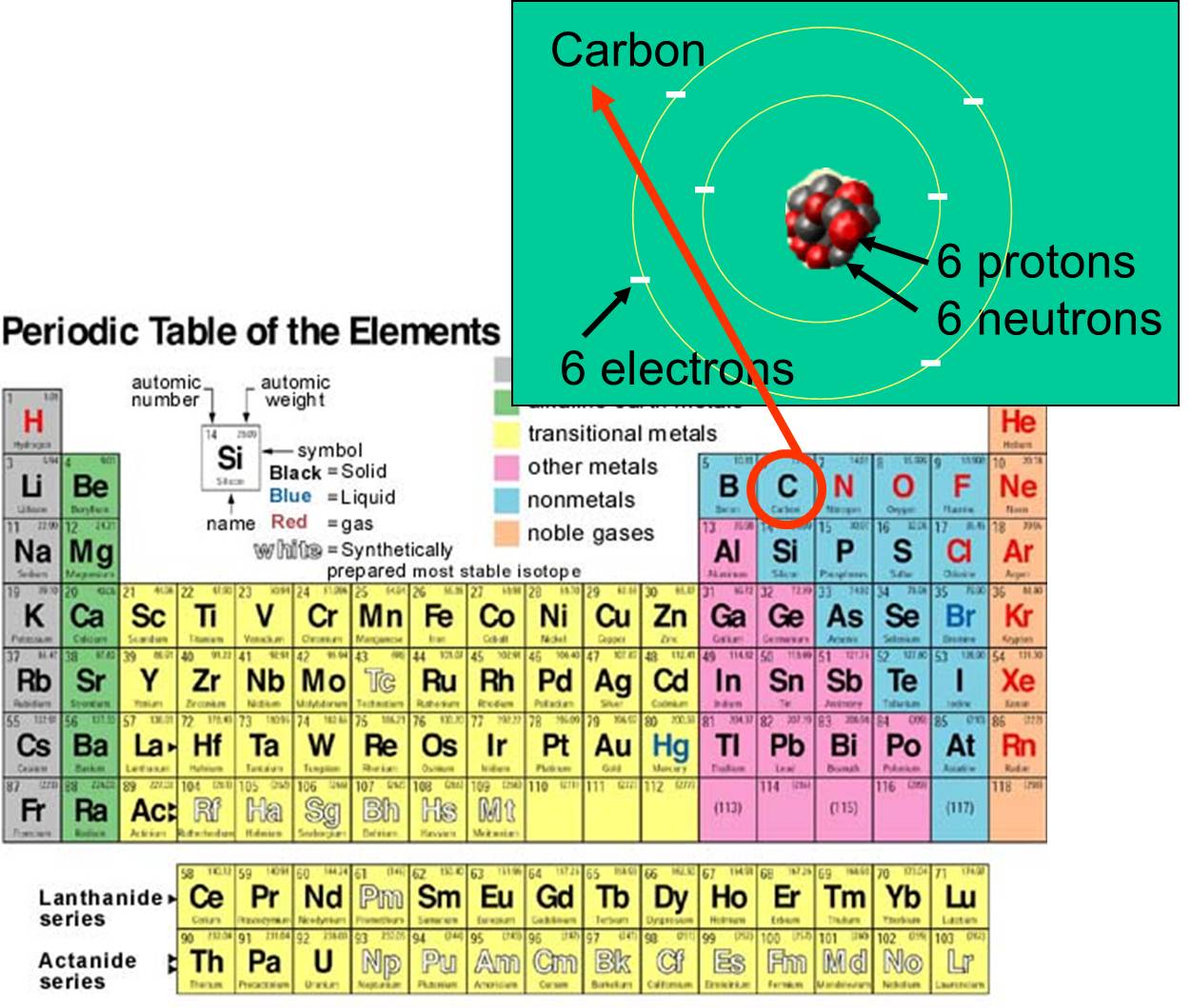
Chemical Elements Atoms
http://sphweb.bumc.bu.edu/otlt/MPH-Modules/PH/PH709_BasicCellBiology/PeriodicTable.jpg
The Element Lithium Has 3 Protons And 4 Neutrons - As seen on a periodic table Lithium has an atomic mass of 7 We know the proton number is 3 so 7 3 4 is our number of neutrons Finally as an atom is neutral in charge the protons