Summary History Of The Atomic Model B Worksheet Answers Atomic History Worksheet Answer Key The document summarizes key discoveries and models in the development of atomic theory including Democritus originally proposed the idea of atoms but lacked evidence while Dalton later established
John Dalton thought that all matter was made of tiny particles called atoms which he imagined as tiny spheres that could not be divided J J Thomson discovered the electron He suggested a plum pudding model of the atom In this model the atom is a ball of positive charge with negative electrons randomly distributed within it Explore atomic models with this worksheet covering Bohr Dalton Rutherford Thomson Democritus and quantum models
Summary History Of The Atomic Model B Worksheet Answers
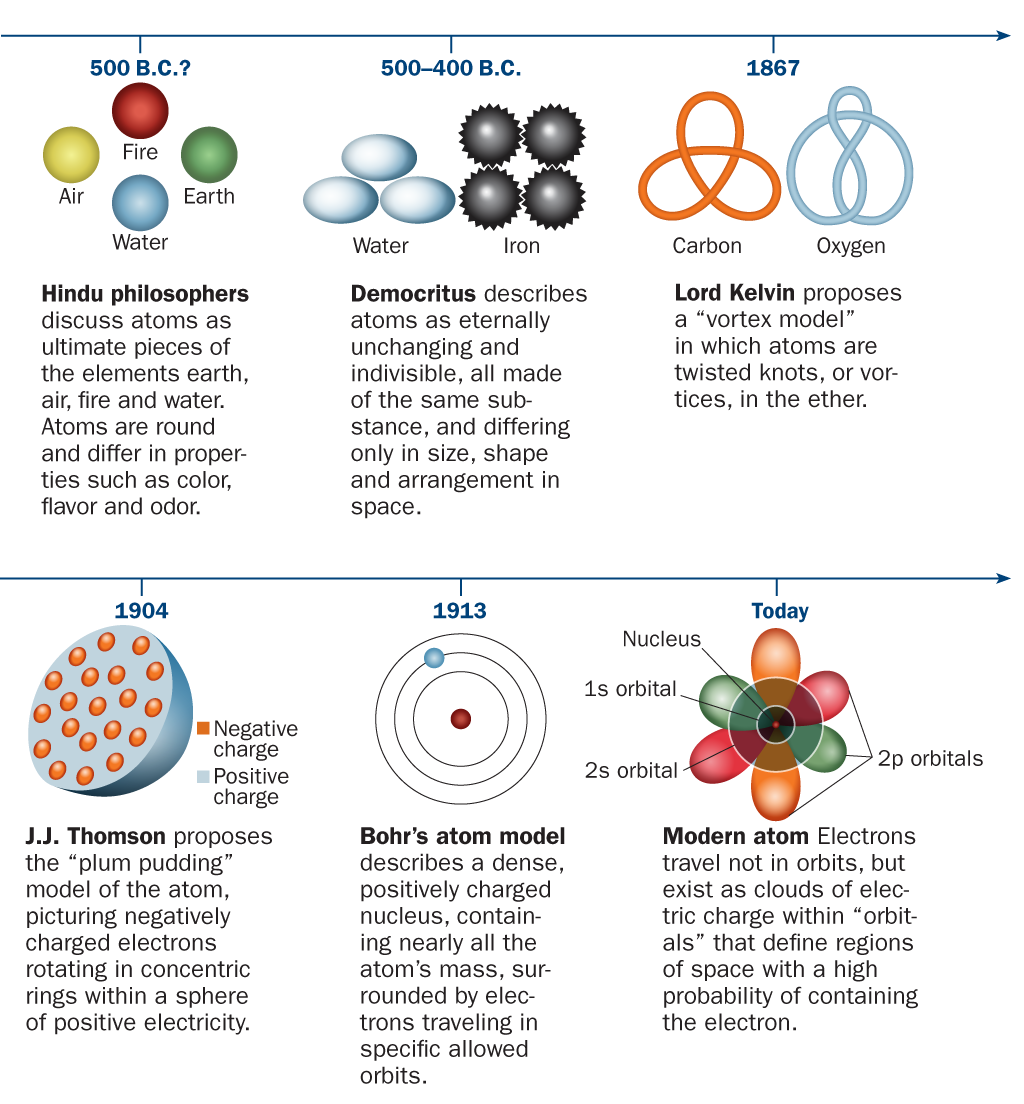
Summary History Of The Atomic Model B Worksheet Answers
https://66.media.tumblr.com/2d5631eeedebfd433f338d7879ac0590/tumblr_mp9hhtriqd1rhb9f5o2_r1_1280.png
Evolution Of The Atomic Model Earth How
https://earthhow.com/wp-content/uploads/2024/01/Evolution-of-the-Atomic-Model.jpg
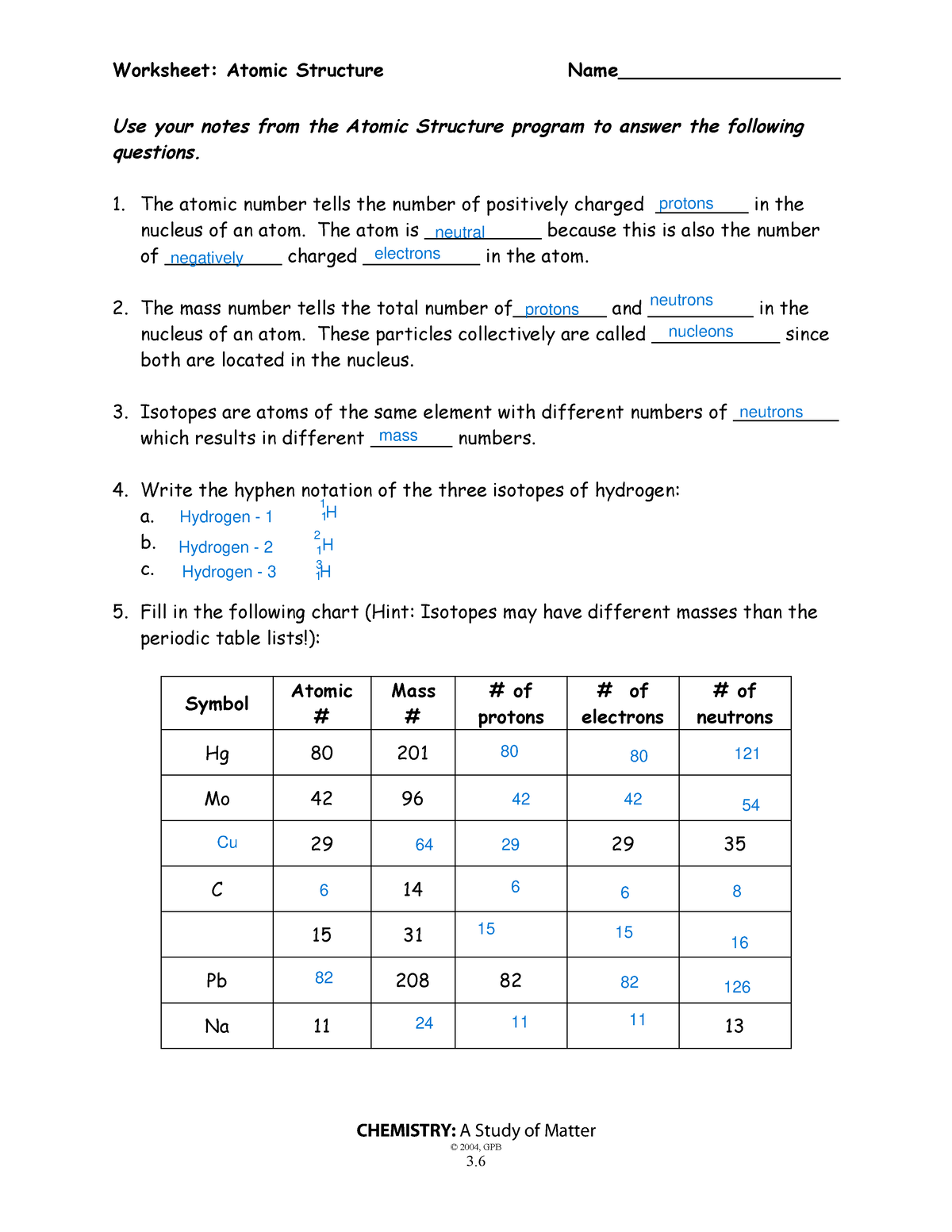
Atomic Structure Wkst Science Worksheet Atomic Structure Name
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/11eec62eecdd0974de2c356a280bc68f/thumb_1200_1553.png
John Dalton was an English chemist His ideas form the atomic theory of matter Here are his ideas All elements are composed made up of atoms It is impossible to divide or destroy an atom All atoms of the same elements are alike One atom of oxygen is like another atom of oxygen Atoms of different elements are different State the most important difference between Dalton s and Thomson s model of the atom Thomson recognized that the atom was very small like Dalton but he proposed that the atom has regions of different charges
Why is Rutherford s experiment called the gold foil experiment It was discovered where a series of landmark experiments were discovered such as the modern model of the atom How did he know that an atom was mostly only made up of empty space Most of the alpha particles passed straight through the foil without being deflected Identify who was the first person to propose the idea or make the discovery Each scientist may be used more than once a Atoms cannot be created destroyed or divided into smaller particles b Discovered the nucleus Rutherford c Electrons occupy specific energy levels or shells
More picture related to Summary History Of The Atomic Model B Worksheet Answers

History Of The Atom Worksheet Pro Worksheet
https://static.docsity.com/documents_first_pages/2021/04/20/df311d69bcef82497bc46f53b3161b61.png
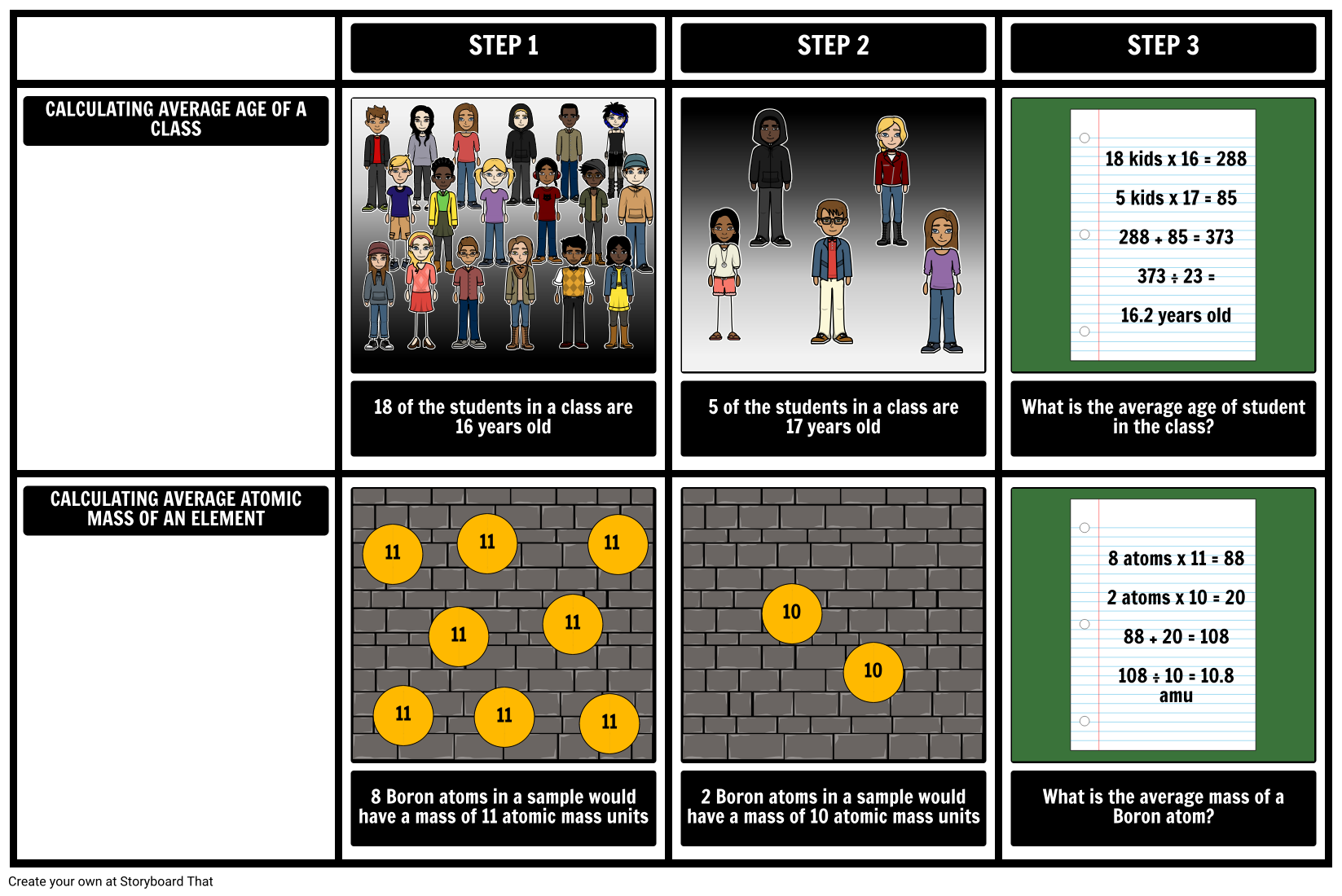
What Is Atomic Mass Storyboard By Amy roediger
https://sbt.blob.core.windows.net/storyboards/amy-roediger/what-is-atomic-mass-.png

Articles The Curious Atom
https://static.wixstatic.com/media/f48c84_26512791ed4e40f18fb28f340fdc532d~mv2.png/v1/fit/w_2500,h_1330,al_c/f48c84_26512791ed4e40f18fb28f340fdc532d~mv2.png
John Dalton was an English chemist His ideas form the atomic theory of matter Here are his ideas All elements are composed made up of atoms It is impossible to divide or destroy an Atomic History Worksheet Name Complete the following Summary table with the contribution of each scientist and the Atomic Model they were using Plum Pudding Solid Spheres Bohr Model Nuclear Model or Quantum Mechanics
History of Atomic Theory Worksheet Q 1 Fill in the blanks with suitable words a The word atom comes from a Greek word that means b Dalton deduced that all elements are composed of c discovered that there were small particles inside the atom Democritus first suggested the existence of the atom but it took almost two millennia before the atom was placed on a solid foothold as a fundamental chemical object by John Dalton 1766 1844 Many unexplained chemical phenomena were quickly explained by Dalton with his theory

The Evolution Of The Atomic Model YouTube
https://i.ytimg.com/vi/W_IujK1yOrU/maxresdefault.jpg

September 1 And September 2 Warm up Ppt Download
https://slideplayer.com/slide/16926242/97/images/2/Agenda+History+of+Atomic+Models.jpg
Summary History Of The Atomic Model B Worksheet Answers - Identify who was the first person to propose the idea or make the discovery Each scientist may be used more than once a Atoms cannot be created destroyed or divided into smaller particles b Discovered the nucleus Rutherford c Electrons occupy specific energy levels or shells