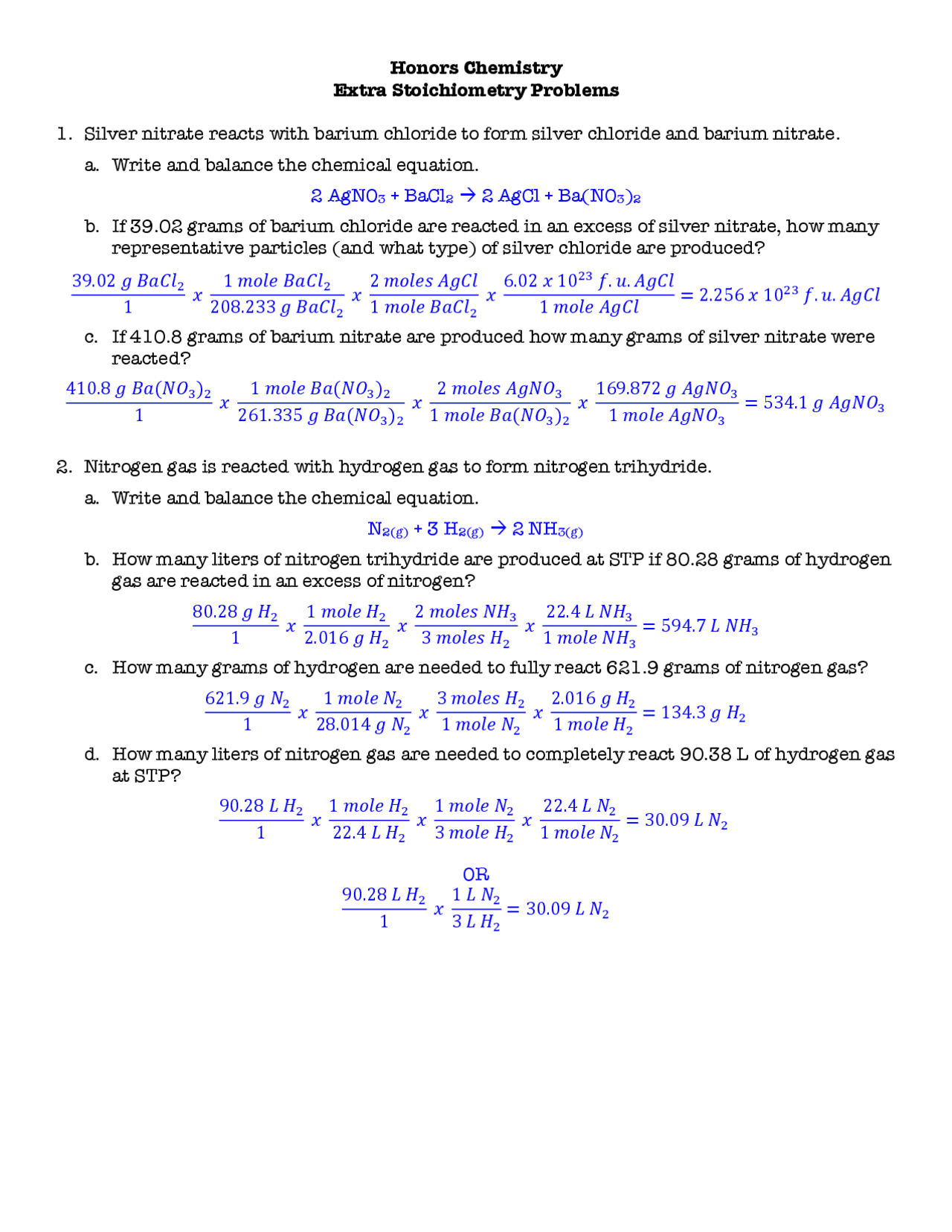
Stoichiometry Worksheet Answers Chemistry Stoichiometry Problem Sheet 1 Directions Solve each of the following problems Show your work including proper units to earn full credit 1 Silver and nitric acid react according to the following balanced equation 3 Ag s 4 HNO 3 aq 3 AgNO 3 aq 2 H 2 O l NO g A
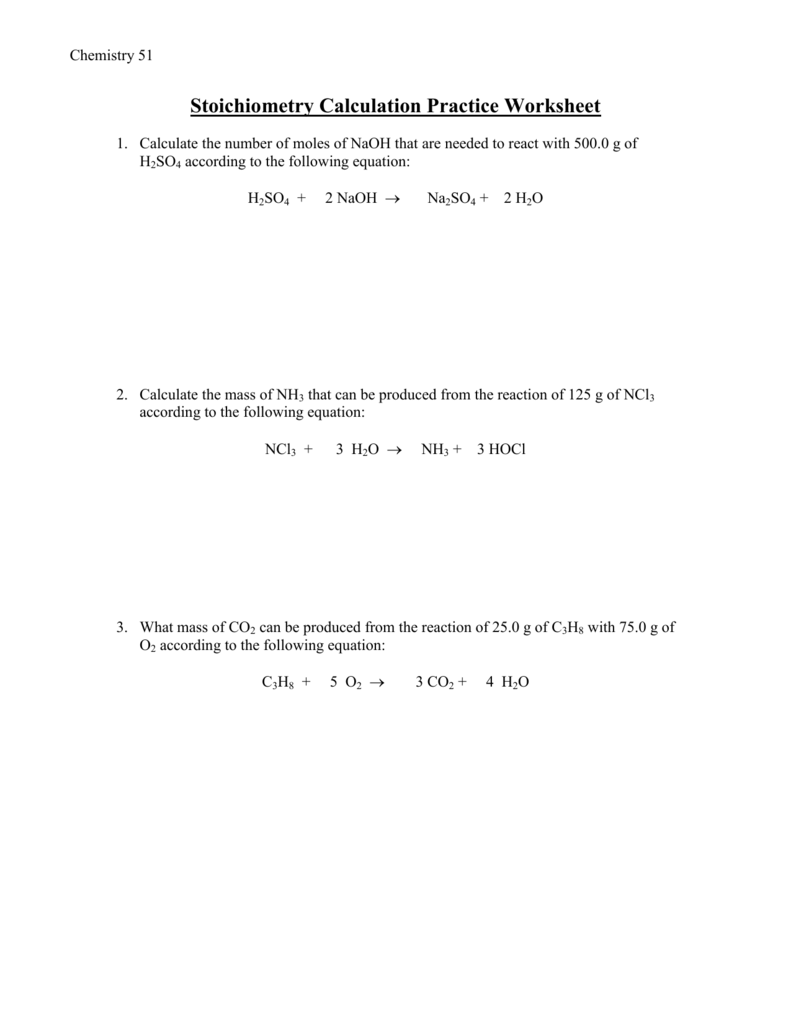
Stoichiometry Calculation Practice Worksheet 1 Calculate the number of moles of NaOH that are needed to react with 500 0 g of H 2 SO 4 according to the following equation H 2 SO 4 2 NaOH Na 2 SO 4 2 H 2 O ANS 10 19 mol 2 Calculate the mass of NH 3 that can be produced from the reaction of 125 g of NCl 3 according to the following equation Mar 4 2013 nbsp 0183 32 When one mole of propane burns in oxygen to produce carbon dioxide and water a ratio of 3 moles of carbon dioxide is produced for every 4 moles of water Determine the formula for propane and write the balanced equation How many L of carbon dioxide gas would be produced STP if 3 4 moles of propane are burned
Stoichiometry Worksheet Answers
Stoichiometry Worksheet Answers
https://static.docsity.com/documents_first_pages/2021/04/20/374813fc83f05cecfbdbe5809326f2b0.png
13 Best Images Of Pressure Problems Worksheet Answer Key
http://www.worksheeto.com/postpic/2015/09/stoichiometry-worksheet-answers_223433.jpg
Stoichiometry Problem Set ANSWERS pdf
https://imgv2-1-f.scribdassets.com/img/document/422639516/original/691db7e186/1589529910?v=1
A How many moles of O2 are required to produce 8 2 moles of H2O b How many moles of H2O are produced when 10 5 moles of O2 react c How many moles of C2H2 are required to produce 3 6 moles of H2O d How many molecules of CO2 are produced when 7 4 moles of C2H2 burn completely in oxygen STEP 1 Balance the reaction Show which compounds are aq and which are s IV FIND LIMITING REACTANT COMBUSTION You burn 35 2 g pentanol C5H12O in 44 8 L of oxygen gas O2 at STP What is the volume of water vapor formed STEP 3a Use your first molar ratio to determine which reactant is limiting Once you have figured this out
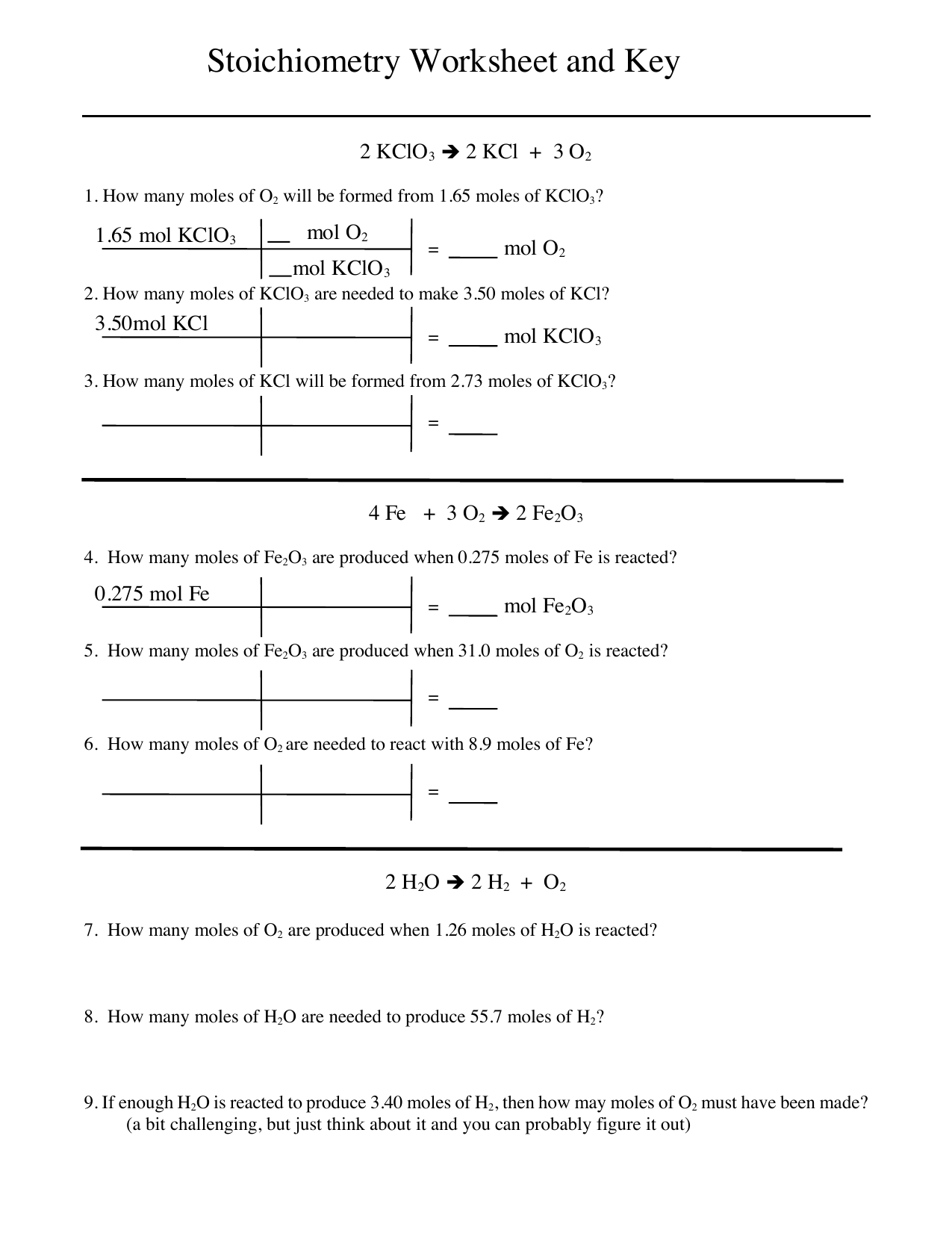
Stoichiometry WorkSheet 1 Worked Solutions Answer the following questions on your own paper Show all work Circle the final answer giving units and the correct number of significant figures 1 Based on the following equation how many moles of each product are produced when 5 9 moles of Zn OH 2 are reacted with H 3 PO 4 You need Stoichiometry with Solutions Name 1 H3PO4 3 NaOH gt Na3PO4 3 H2O How much 0 20 M H3PO4 is needed to react with 100 ml of 0 10 M NaOH 2 2 HCl Zn gt ZnCl2 H2 When you use 25 ml of 4 0 M HCl to produce H2 gas how many grams of zinc does it react with What volume of H2 gas is produced at STP 3
More picture related to Stoichiometry Worksheet Answers

22 Solutions Stoichiometry Practice Problems With Answers Balance The
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/d97b361d7613b0927e86906c0a877bab/thumb_1200_1553.png
Stoichiometry 1 Worksheet And Key
https://s3.studylib.net/store/data/025349974_1-ab754f5a90c0fe64b187b19524c5aea0.png
Stoichiometry Practice 2 Worksheet Answers Koyumprogram
https://s3.studylib.net/store/data/008265920_1-9997ec2073d9fc7388e53f6adce5ae19.png
An answer key is provided with the solutions to each problem This document is a stoichiometry worksheet containing 10 chemistry problems involving mole mole calculations determining limiting reagents and calculating theoretical and percent yields in chemical reactions This document contains 10 chemistry problems involving stoichiometric calculations using balanced chemical equations The problems require determining molar ratios moles or masses of reactants and products The document tests understanding of mole mole and mass mass stoichiometric conversions
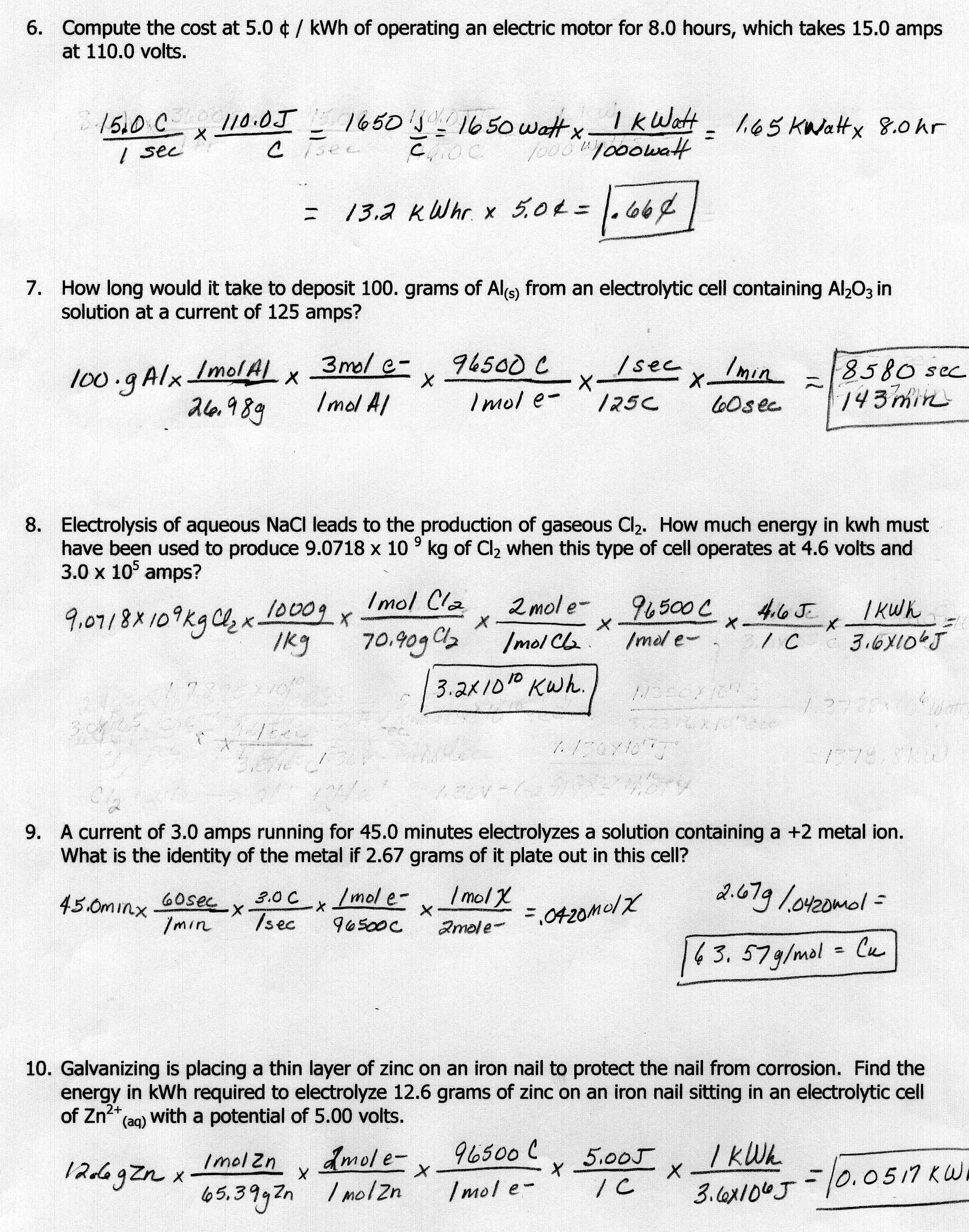
Chemistry Molarity and Stoichiometry Directions Using the definition of molarity the given balanced equations and stoichiometry solve the following problems Show your work and include units for full credit 1 Calcium hydroxide slaked lime and sulfuric acid react to produce calcium sulfate and water according to GAS STOICHIOMETRY WORKSHEET Please answer the following on separate paper using proper units and showing all work Please note that these problems require a balanced chemical equation 1 Carbon monoxide reacts with oxygen to produce carbon dioxide I f 1 0 L of carbon monoxide reacts with oxygen at STP a

Chemistry Stoichiometry Worksheet 1
https://tempojs.com/wp-content/uploads/2016/10/education-essay-day-005-stoichiometry-worksheet-1-blank-facts-stoichiometry.jpeg

Solution Stoichiometry Worksheet Key Studocu
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/de00ed5bb73cdab7c5fcad3318aaf2d8/thumb_1200_1553.png
Stoichiometry Worksheet Answers - 1 How many grams of calcium phosphate can be produced from the reaction of 2 50 L of 0 250 M Calcium chloride with and excess of phosphoric acid 4 For the following equation determine which reactant is the limiting reactant and which reactant is in excess The amounts of reagent used are shown
