Stoichiometry Mass To Mass Worksheet Answers Chemistry Stoichiometry Problem Sheet 1 Directions Solve each of the following problems Show your work including proper units to earn full credit 1 Silver and nitric acid react according to the following balanced equation 3 Ag s 4 HNO 3 aq 3 AgNO 3 aq 2 H 2 O l NO g A
Problem 1 Given the following equation 2C 4 H 10 13O 2 gt 8CO 2 10H 2 O Show what the following molar ratios should be a C 4 H 10 O 2 b O 2 CO 2 c O 2 H 2 O d C 4 H 10 CO 2 e C 4 H 10 H 2 O Solution Stoichiometry Worksheet 1 Answers 1 Given the following equation 2 C4H10 13 O2 gt 8 CO2 10 H2O show what the following molar ratios should be a C4H10 O2 b O2 CO2 c O2 H2O d C4H10 CO2 e C4H10 H2O 2 Given the following equation 2 KClO3
Stoichiometry Mass To Mass Worksheet Answers

Stoichiometry Mass To Mass Worksheet Answers
https://images.sampletemplates.com/wp-content/uploads/2016/11/17162322/Mass-to-Mass-Stoichiometry-Worksheet.jpg

13 Best Images Of Molar Mass Practice Worksheet Answers Mole
http://www.worksheeto.com/postpic/2013/10/mole-calculation-worksheet-answer-key_708267.jpg
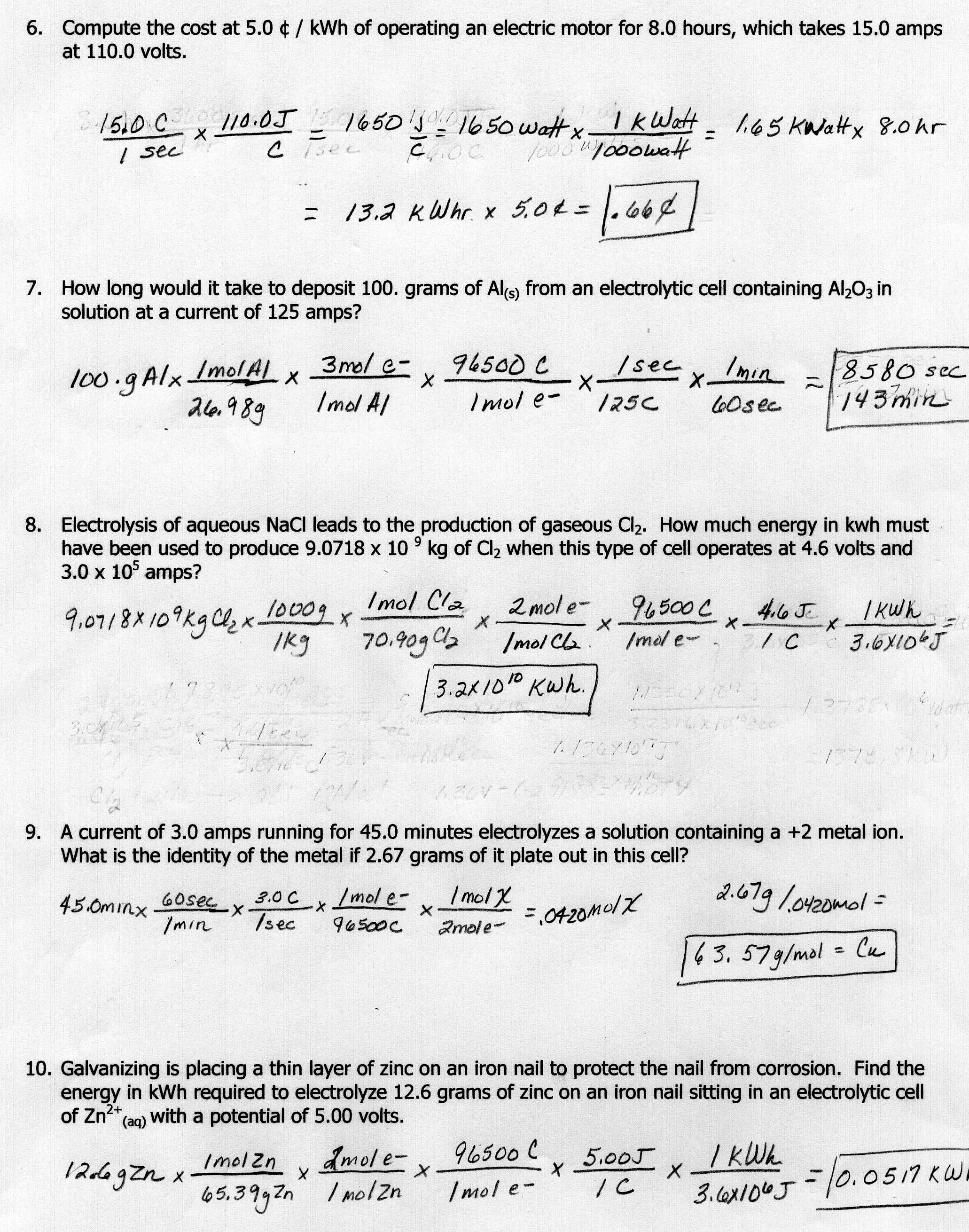
8 Best Images Of Mole Ratio Worksheet How To Graph Absorbance Mole
http://www.worksheeto.com/postpic/2012/06/stoichiometry-worksheet-answers_670813.jpg
Sep 21 2022 nbsp 0183 32 Mass mass calculations involve converting the mass of a reactant to moles of reactant then using mole ratios to determine moles of product which can then be converted to mass of product Mass to Mass Stoichiometry Problems Answer Key In the following problems calculate how much of the indicated product is made Show all your work If you start with ten grams of lithium hydroxide how many grams of lithium bromide will be produced If you start with 45 grams of ethylene C2H4 how many grams of carbon dioxide will be
Answer key for mass to mass stoichiometry practice problems Stoichiometry WorkSheet 1 Worked Solutions Answer the following questions on your own paper Show all work Circle the final answer giving units and the correct number of significant figures 1 Based on the following equation how many moles of each product are produced when 5 9 moles of Zn OH 2 are reacted with H 3 PO 4 You need
More picture related to Stoichiometry Mass To Mass Worksheet Answers
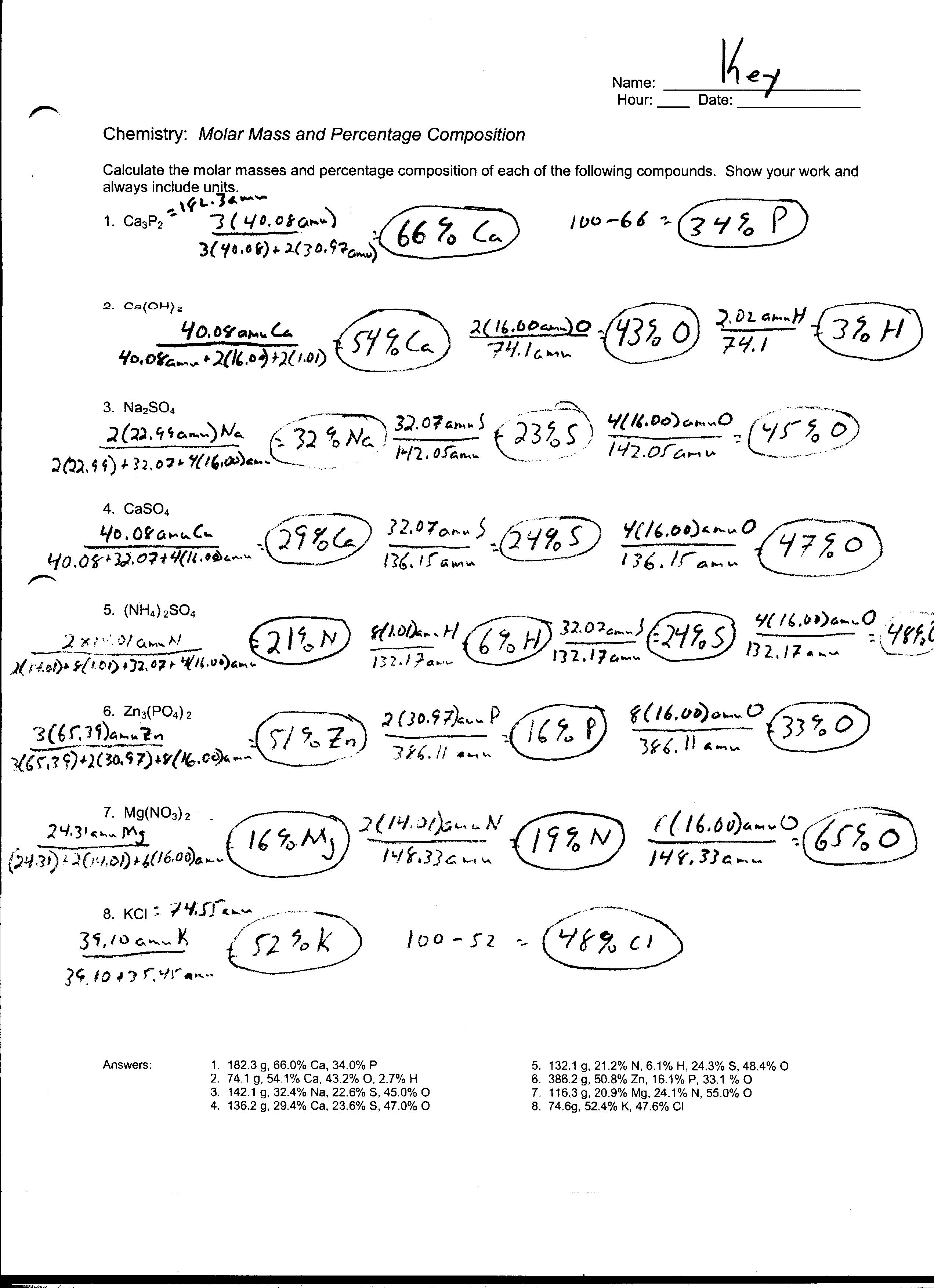
5 Best Images Of Chemistry If8766 Worksheet Answers Mass To Mole
http://www.worksheeto.com/postpic/2013/06/mass-to-mole-stoichiometry-worksheet-answer-key_708283.jpg

Stoichiometry Mass To Mass Practice 3 YouTube
https://i.ytimg.com/vi/WRA7-aKs3Yk/maxresdefault.jpg
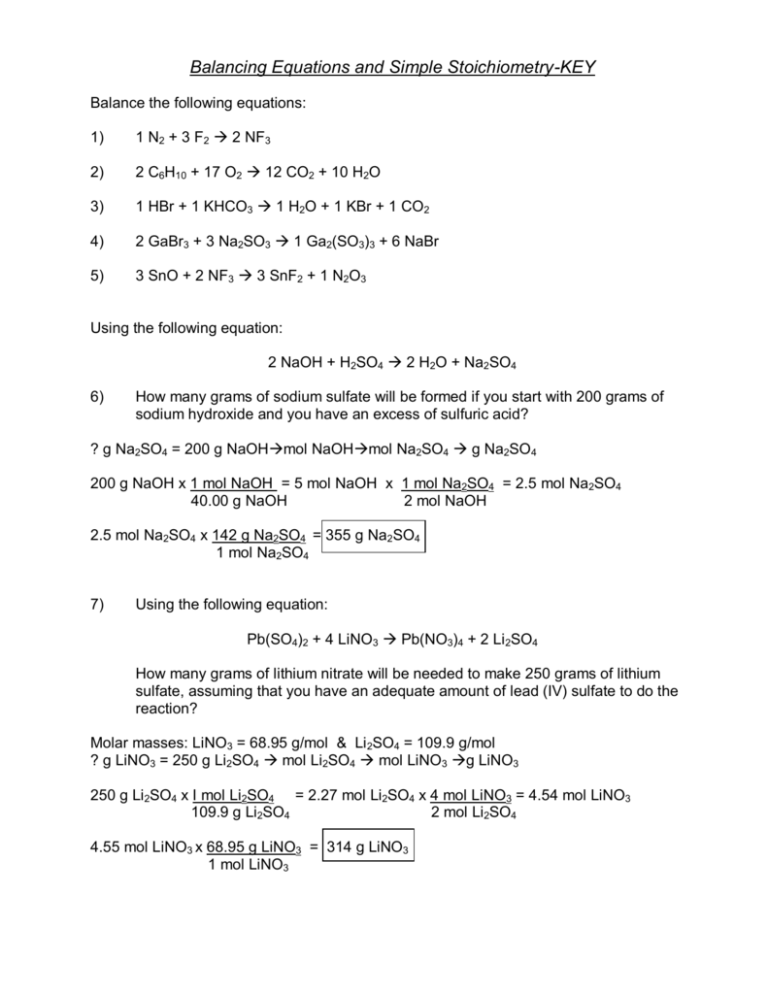
KEY Solutions For The Stoichiometry Practice Worksheet
https://s3.studylib.net/store/data/008711874_1-d12f29b6b62688faeae95fc3bd7c9543-768x994.png
Question Answer Methane burns readily in oxygen to produce carbon dioxide and water according to the equation CH4 g 2O2 g CO2 g 2H2O l How many mol of CO2 will be produced by the complete oxidation of 4 5 mol of CH4 in excess oxygen 2 We can use that relation to answer stoichiometry questions in terms of the masses of a particular substance in addition to moles We do this using the following sequence moles of A molar ratio moles of B molar mass grams of B Collectively these conversions are called mole to mass calculations
[desc-10] [desc-11]
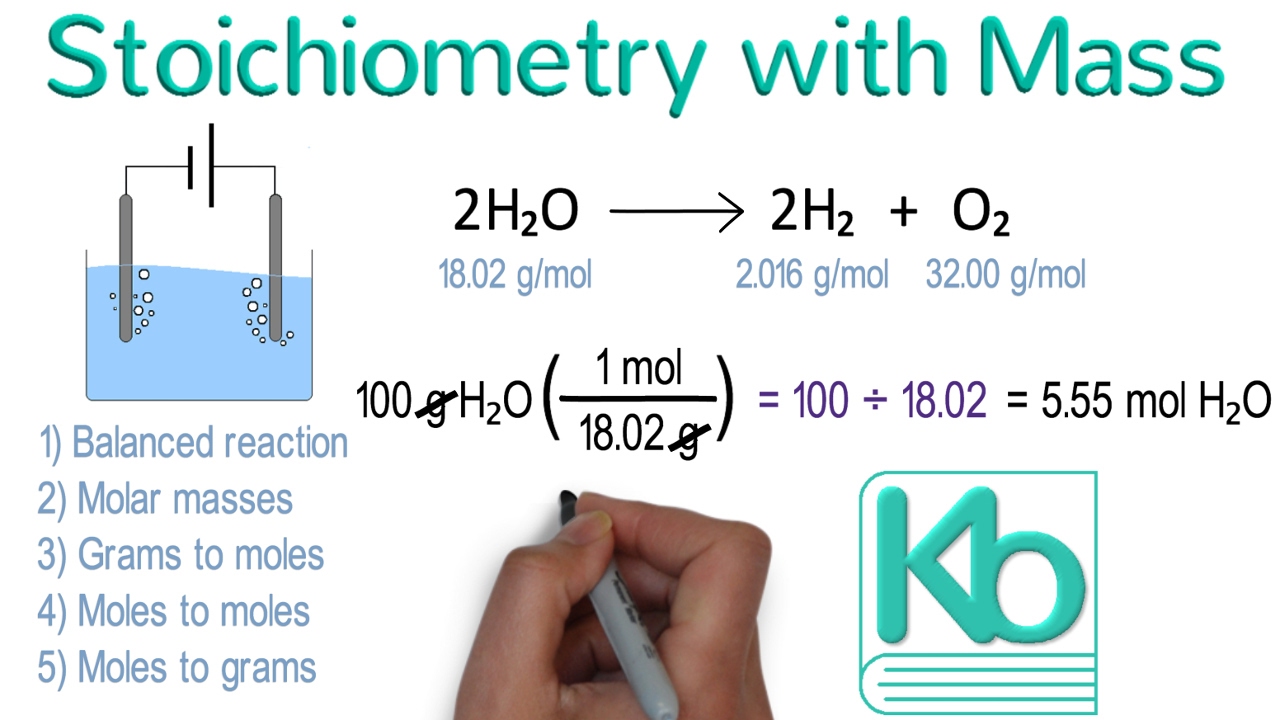
Stoichiometry With Mass Stoichiometry Tutorial Part 2 YouTube
https://i.ytimg.com/vi/BZuS3Agn4pI/maxresdefault.jpg

Examples Of Mass To Mass Stoichiometry Problems YouTube
https://i.ytimg.com/vi/BMPu3a9K0Zo/maxresdefault.jpg
Stoichiometry Mass To Mass Worksheet Answers - Stoichiometry WorkSheet 1 Worked Solutions Answer the following questions on your own paper Show all work Circle the final answer giving units and the correct number of significant figures 1 Based on the following equation how many moles of each product are produced when 5 9 moles of Zn OH 2 are reacted with H 3 PO 4 You need