Specific Heat Capacity And Specific Latent Heat Worksheet How much heat is absorbed by 100 g of ice at 10 C to become water at 20 C 2 A 200 g sample of water at 80 C is heated to steam at 120 C How much heat does it absorb 3 How much heat is needed to change 2 kg of ice at 50 C into steam at 150 C 4 An ice chest is filled with 10 lb 4 5 kg of ice at 0 C
1 What does it mean if water has a higher speci c heat capacity than oil It requires more energy to raise its temperature by the same amount 2 What is the difference between thermal energy and temperature Temperature is a measure of the average kinetic energy of the molecules present Thermal Specific Latent Heat worksheets questions and revision for GCSE Combined Science and Physics All the revision you need in one place
Specific Heat Capacity And Specific Latent Heat Worksheet

Specific Heat Capacity And Specific Latent Heat Worksheet
https://i.ytimg.com/vi/KyC9bZNSujY/maxresdefault.jpg

Specific Latent Heat And Heat Capacity Exam Question YouTube
https://i.ytimg.com/vi/sXnmvJNsmYU/maxresdefault.jpg

Specific Heat Capacity And Latent Heat Calculations YouTube
https://i.ytimg.com/vi/zvr-iuJxRYw/maxresdefault.jpg
Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24 5oC to 26 4oC How much heat did this sample absorb c for water 4 18 J goC ans 2 66 kJ 2 How much heat in kilojoules has to be removed from 225g of water to lower its temperature from 25 0oC to 10 0oC ans 14 1 kJ 3 As the value for specific heat capacity increases more energy is needed to raise the temperature of a certain mass of substance Good conductors such as metals have low specific heat capacity Only a small amount of energy is needed to raise the temperature of a metal
The specific latent heat of vaporisation is the energy required to change 1 kg of liquid into gas As we have just discussed changing from a liquid to a gas takes more energy than changing a solid into a gas so Sep 11 2024 nbsp 0183 32 A Level Physics worksheet containing questions about the lesson Specific Heat Capacity amp Latent Heat Model Answers Included This worksheet covers the following content specific heat capacity of a substance the equation E mc T an electrical experiment to determine the specific heat capacity of a metal or a liquid specific latent heat of
More picture related to Specific Heat Capacity And Specific Latent Heat Worksheet

Specific Heat Worksheet Answers Imsyaf
https://static.docsity.com/documents_first_pages/2021/04/20/20a00d187ca99eee26a6962477a81d43.png
New AQA GCSE Trilogy physics Specific Heat Capacity And Latent Heat
https://d1e4pidl3fu268.cloudfront.net/bd5dc91b-f47e-4e43-927d-f1a361246003/Slide12.JPG

Specific Latent Heat Notes And Video Lesson The Fizzics Organization
https://www.fizzics.org/wp-content/uploads/2021/12/Screenshot-2021-12-09-at-17.42.32.png
DIRECTIONS Use q m T Cp to solve the following problems Show all work and units 1 A 15 75 g piece of iron absorbs 1086 75 joules of heat energy and its temperature changes from 25 176 C to 175 176 C Calculate the specific heat capacity of iron 2 Calculate the specific heat capacity of iron How many joules of heat are needed to raise the temperature of 10 0 g of aluminum from 22 176 C to 55 176 C if the specific heat of aluminum is 0 90 J g 176 C
Jun 11 2022 nbsp 0183 32 Specific heat capacity is defined as the energy required to raise the temperature of one kilogram of a substance by one kelvin The increase in temperature of an object depends on The amount of heat energy transferred The mass of the object The specific heat capacity of the material from which the object is made Explanation of Heat Energy Here are the heat capacities of the four substances 4 18 J g 176 c 1 00 J g 176 c 0 80 J g 176 c amp 0 60 J g 176 c Match amp then label each substance with its specific heat capacity on the graph 7 If something has a high specific heat capacity will it take a lot of heat or a little heat to change its temperature Explain careful
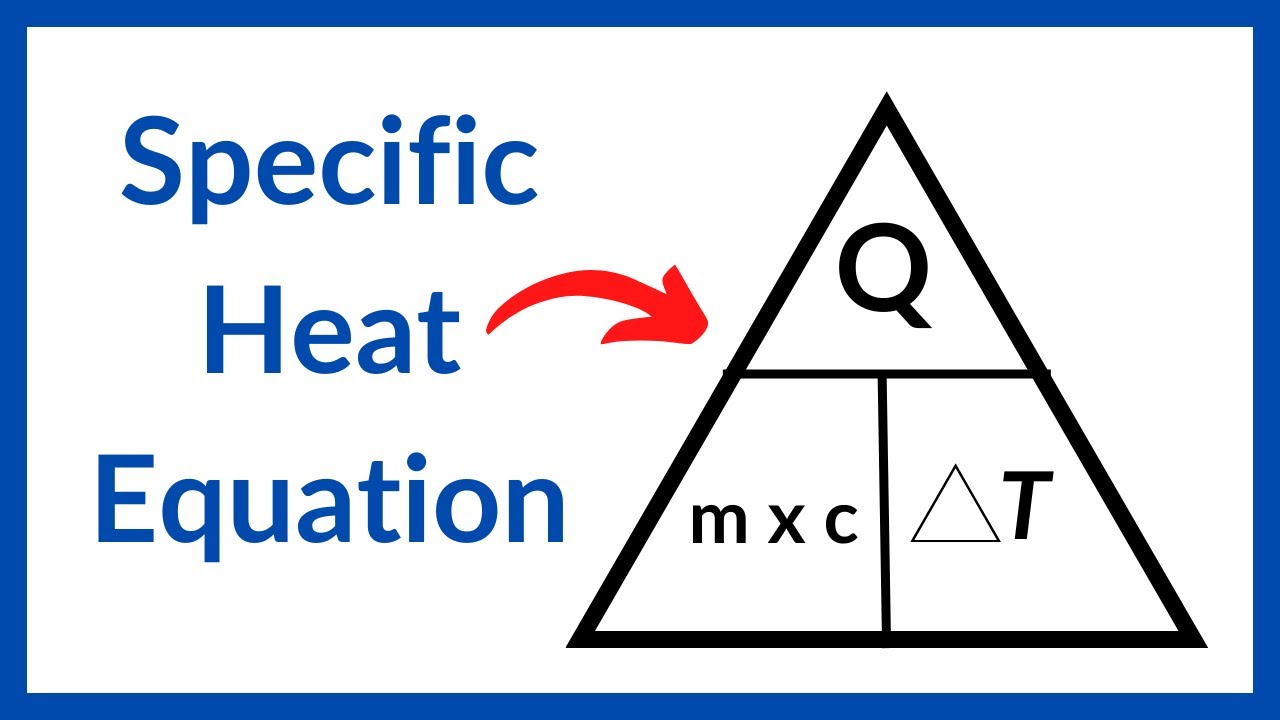
Specific Heat Equation Stated Clearly YouTube
https://i.ytimg.com/vi/uRQ8OjSj1lg/maxresdefault.jpg

Changes Of Heat And Specific Latent Heat YouTube
https://i.ytimg.com/vi/NMGkFa0_H_4/maxresdefault.jpg
Specific Heat Capacity And Specific Latent Heat Worksheet - The specific latent heat of vaporisation is the energy required to change 1 kg of liquid into gas As we have just discussed changing from a liquid to a gas takes more energy than changing a solid into a gas so
