Rate Of Reaction Igcse Oct 22 2024 nbsp 0183 32 Explaining rates of reaction Increasing the number of successful collisions means that a greater proportion of reactant particles collide to form product molecules We have seen previously that the following factors
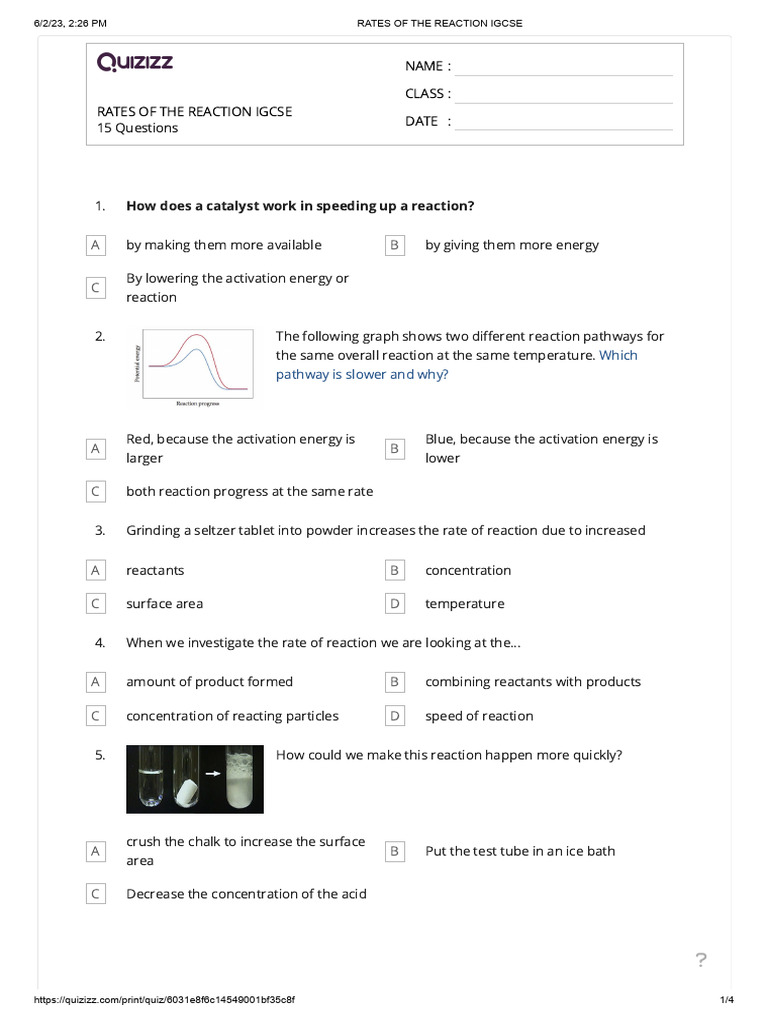
Summary notes flashcards and past exam questions by topic for CAIE IGCSE Chemistry Topic 6 Chemical Reactions Rate of reaction Quantity of products formed Time taken s Quantity of reactants used Time taken s Rate is a measure of the change that happens in a single unit of time
Rate Of Reaction Igcse
Rate Of Reaction Igcse
https://imgv2-2-f.scribdassets.com/img/document/681747138/original/2bf1feaba2/1702018164?v=1
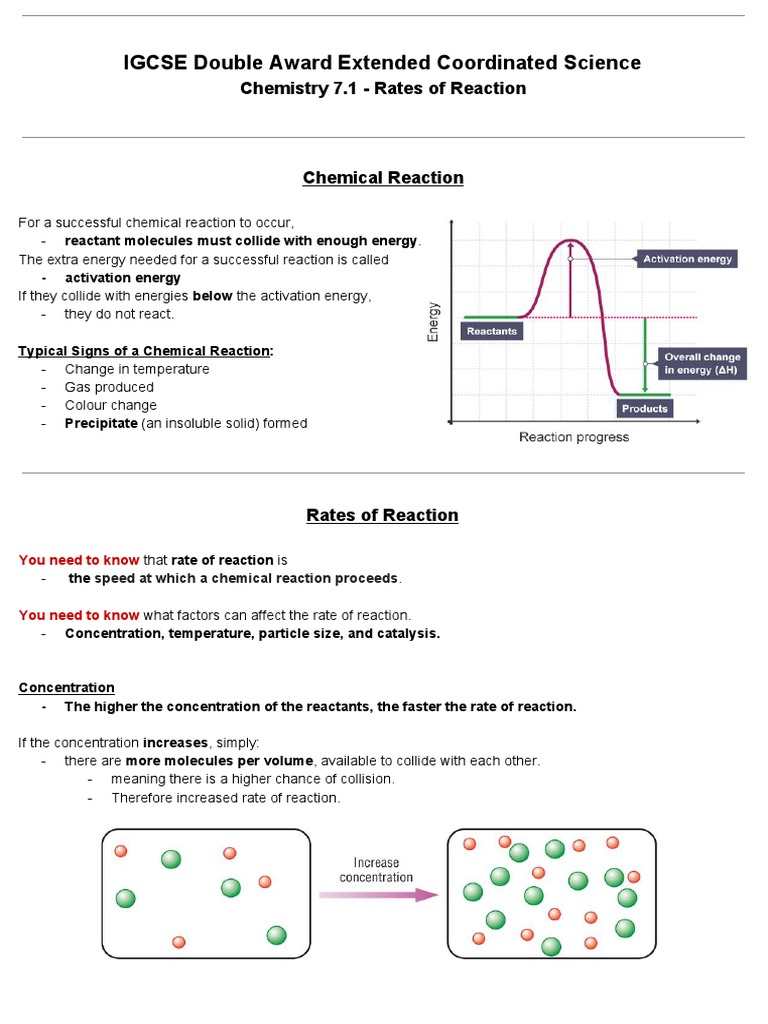
IGCSE Double Award Extended Coordinated Science Chemistry 7 1 Rates
https://imgv2-1-f.scribdassets.com/img/document/570133185/original/d18412075f/1707425896?v=1
Questions Rate Of Reaction IGCSE Chemistry Lesson 19 Part A YouTube
https://i.ytimg.com/vi/dMlu1ehPw9c/maxresdefault.jpg
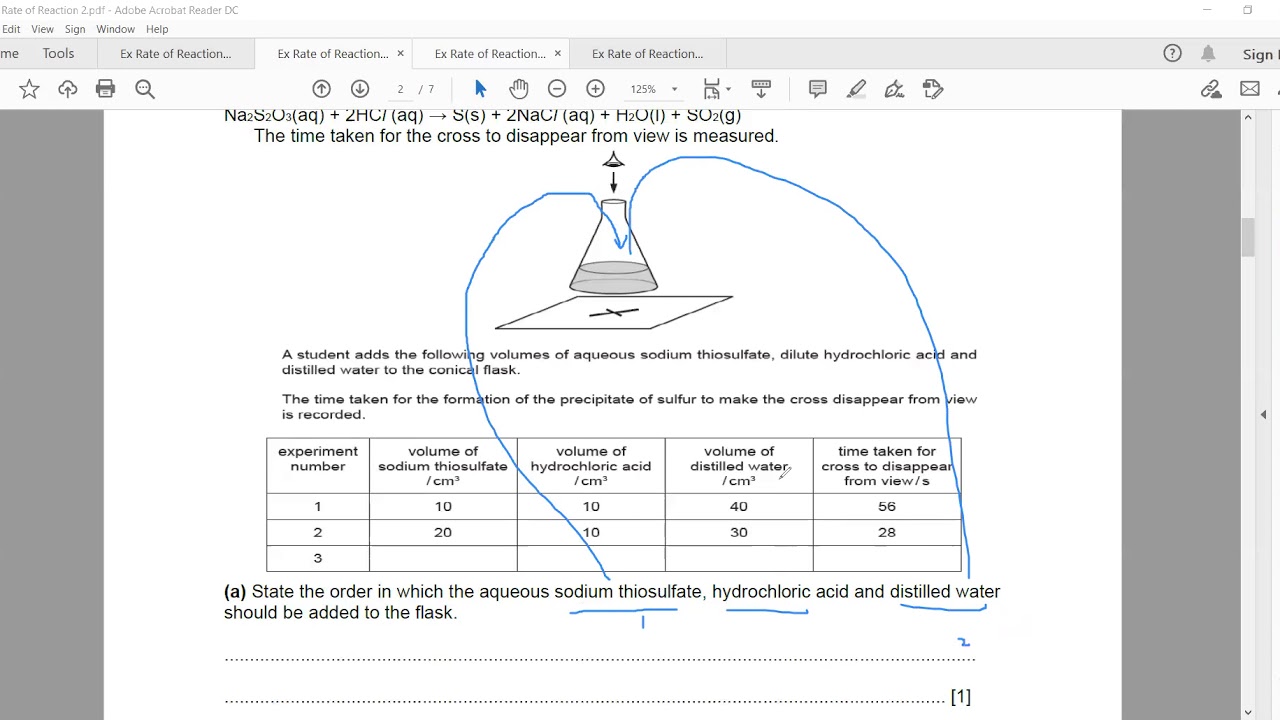
Devise a suitable method for investigating the effect of a given variable on the rate of a reaction You can measure what effect a change in temperature The rate of reaction is simply the speed at which a reaction takes place There are several factors that affect the rate of reaction such as surface area temperature concentration and the catalyst
Rate is a measure of how fast a reaction goes its speed The rate of a chemical reaction is the speed at which the reactants are used up or the speed at which new products are formed How to measure the rate of a chemical reaction and how to interpret this from a graph The rate of a reaction depends on the frequency of collisions between the reactant molecules Show Step
More picture related to Rate Of Reaction Igcse

Rate Of Reaction IGCSE O Level Part 4 YouTube
https://i.ytimg.com/vi/DFvkRrhWfSM/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AGMAoAC4AOKAgwIABABGGUgZShlMA8=&rs=AOn4CLBAgyPCv0jnt1jHKce6r4KhHcrAxA
IGCSE Chemistry Rates Of Reaction YouTube
https://i.ytimg.com/vi/uCaD4-tVR7E/maxresdefault.jpg

GCSE Chemistry Rates Of Reaction 46 YouTube
https://i.ytimg.com/vi/SPXanyy3-hU/maxresdefault.jpg
Discover which factors indicate the rate of a chemical reaction and how you can change it 1 Surface Area of a Solid Materials and equipment 2 glasses 2 soluble aspirin tablets cold The rate of reaction refers to the speed at which reactants are turned into products in a chemical reaction It s usually measured by how much of a reactant is used up per unit of time or how
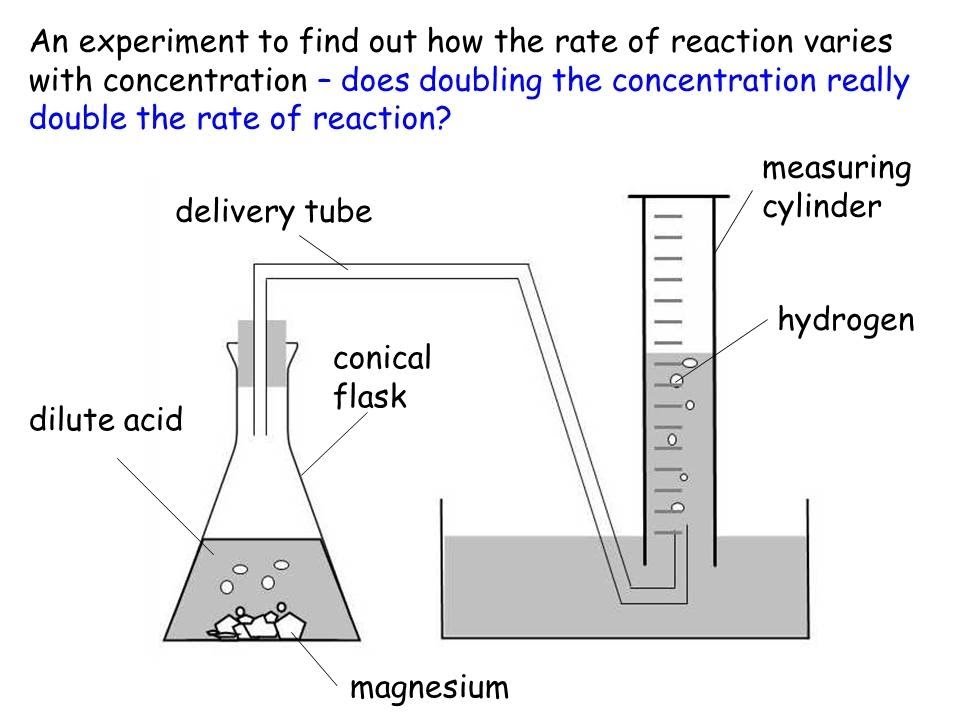
Sep 24 2024 nbsp 0183 32 Investigating the rate of a reaction To measure the rate of a reaction we need to be able to measure How quickly the reactants are used up OR How quickly the products are formed The method used for measuring Increasing the pressure on a reaction between gases will increase the rate of reaction Increasing the pressure reduces the volume of gas moving the particles closer together If the particles
Rate Of Reaction IGCSE Chemistry 24 YouTube
https://i.ytimg.com/vi/ZxLRg3LeVv4/maxresdefault.jpg
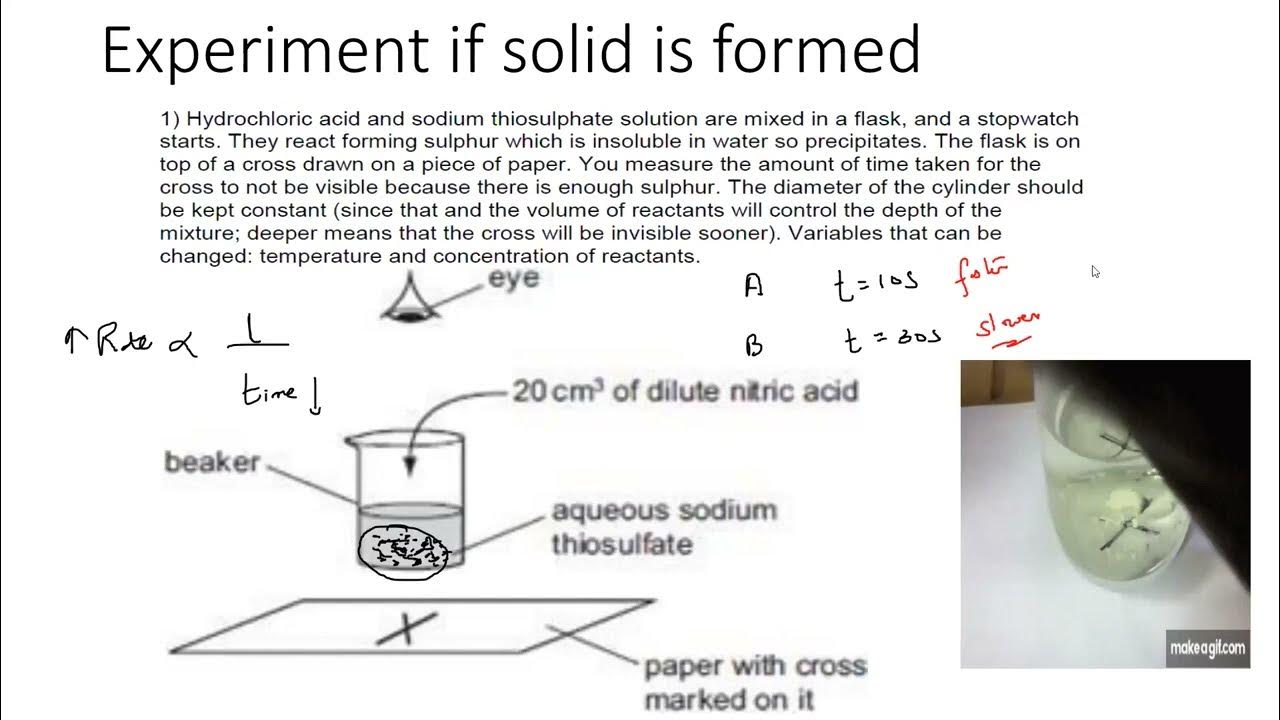
Questions Rate Of Reaction IGCSE O Level Chemistry YouTube
https://i.ytimg.com/vi/4LaMoruT0dI/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLBfffNTgOdhKyNq-i1ZJuCgdWyOAg
Rate Of Reaction Igcse - Rate is a measure of how fast a reaction goes its speed The rate of a chemical reaction is the speed at which the reactants are used up or the speed at which new products are formed