Rate Law And Reaction Order Feb 13 2023 nbsp 0183 32 The reaction order is the relationship between the concentrations of species and the rate of a reaction The order of a rate law is the sum of the exponents of its concentration
Explain the form and function of a rate law Use rate laws to calculate reaction rates Use rate and concentration data to identify reaction orders and derive rate laws A rate law shows how the rate of a chemical reaction depends on reactant concentration For a reaction such as aA products the rate law generally has the form rate k A where k is a
Rate Law And Reaction Order

Rate Law And Reaction Order
https://i.ytimg.com/vi/6Ng8ayarWHw/maxresdefault.jpg

Intro To Rate Laws Rate Constants Reaction Order Chemistry Tutorial
https://i.ytimg.com/vi/2qw8GJUqMMY/maxresdefault.jpg

How To Use The Integrated Second Order Rate Law Equation To Solve For T
https://i.ytimg.com/vi/asTXZPlX_WM/maxresdefault.jpg
Nov 13 2022 nbsp 0183 32 Determine the order of a reaction of the form A B C from experimental data for the concentrations of its products at successive times Describe the initial rate and isolation methods of determining the orders of the In chemistry the rate equation also known as the rate law or empirical differential rate equation is an empirical differential mathematical expression for the reaction rate of a given reaction in
A rate law shows how the rate of a chemical reaction depends on reactant concentration For a reaction such as aA products the rate law generally has the form rate k A where k is a How exactly the rate of a reaction depends on the concentration of reactant s is given by what is called the rate law or differential rate law of the reaction For a simple reaction with one reactant the rate law could be written as A
More picture related to Rate Law And Reaction Order
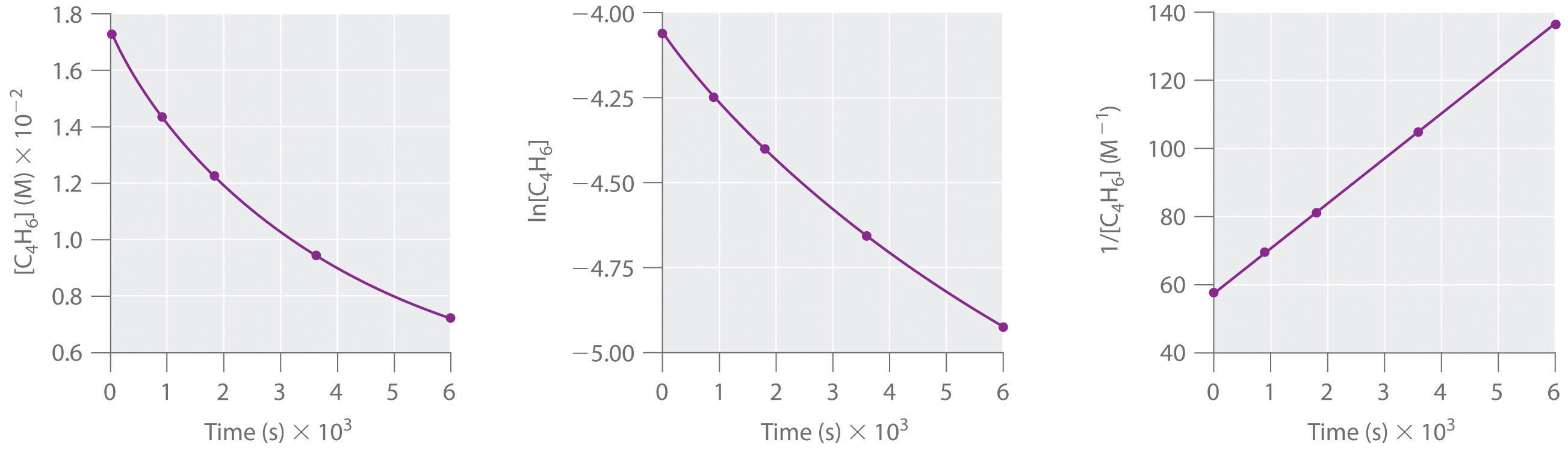
Using Graphs To Determine Rate Laws Rate Constants And Reaction Orders
http://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/section_18/db20fbc24566c56823df39e68c74dd7f.jpg
How To Determine Order Of Reaction From Rate Law Images And Photos Finder
http://www.sliderbase.com/images/referats/167b/(10).PNG
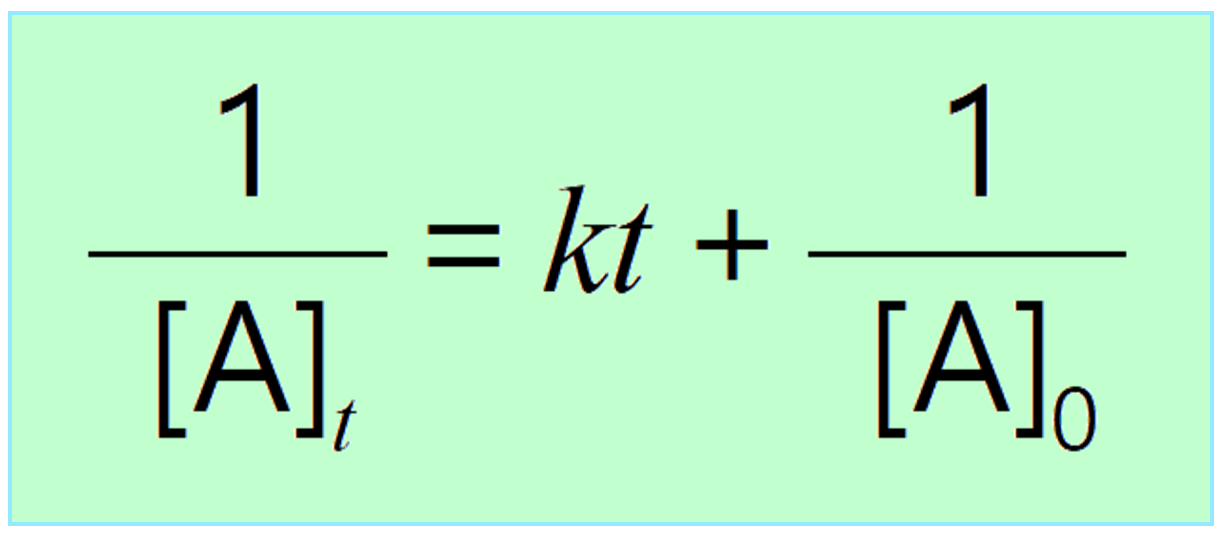
Integrated Rate Law Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/04/Second-order-integrated-rate-law.png
In this article we will learn about reaction rates rate laws the rate constant and the reaction order The rate of a chemical reaction is determined and altered by many factors including the nature of reactivity of reactants The rate law provides a relationship between reaction rates and reactant concentrations Learn about the integrated rate equations amp rate constants for different reaction orders
We need to know the rate law of a reaction in order to determine The order of the reaction with respect to one or more reactants The overall order of the reaction Changing the concentration of substances taking part in a reaction usually changes the rate of the reaction A rate equation shows this effect mathematically Orders of reaction are a part

How To Determine Order Of Reactants Using Initial Rate Method And Write
https://i.ytimg.com/vi/fjqtFbp_6GI/maxresdefault.jpg

Determining The Rate Law Using Initial Rates Data Example Pt 1 Of 3
https://i.ytimg.com/vi/YslxSIOB9h4/maxresdefault.jpg
Rate Law And Reaction Order - A rate law shows how the rate of a chemical reaction depends on reactant concentration For a reaction such as aA products the rate law generally has the form rate k A where k is a
.PNG)