Periodic Trends Worksheet 2 Answers Directions Use your notes to answer the following questions 1 Rank the following elements by increasing atomic radius carbon aluminum oxygen potassium 2 Rank the following
Remember which way the three trends radius ionization energy electronegativity increase on the PT up down left right Using the data below make a bar graph of atomic radius vs atomic number for Group 2A and for Period 3 of the periodic table 2 What trends do you notice for the atomic radii of Group 2A 3
Periodic Trends Worksheet 2 Answers
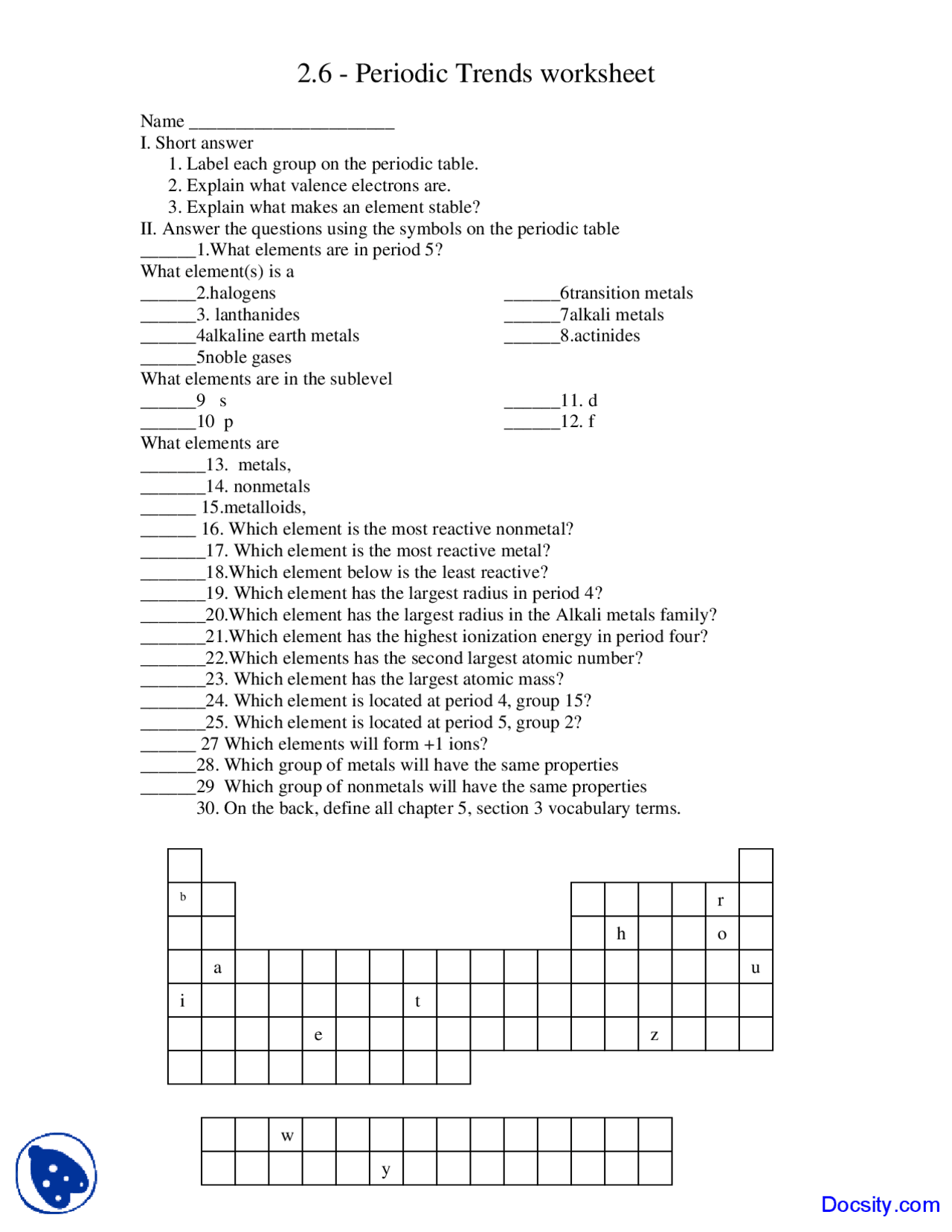
Periodic Trends Worksheet 2 Answers
https://static.docsity.com/documents_first_pages/2013/01/30/fb6ea015d019163e63484e449b0f74ab.png

Periodic Table Trends Worksheet Answers Chemistry A Study Of Matter
https://i1.wp.com/i.pinimg.com/originals/c2/f2/b3/c2f2b319d127b6b98aaccc372befaa69.png?resize=618%2C464&ssl=1

50 Periodic Trends Worksheet Answer Key
https://chessmuseum.org/wp-content/uploads/2019/10/periodic-trends-worksheet-answer-key-lovely-20-best-of-periodic-trends-worksheet-answers-key-of-periodic-trends-worksheet-answer-key.png
In this activity you will look at a few periodic trends that can help you make those predictions Like most trends they are not perfect but useful just the same 1 Consider the data in Model 1 on It provides the answers to questions about classifying elements as metals nonmetals or metalloids It also answers questions about trends in atomic radius ionization energy and electronegativity across the periodic table including
Worksheet Periodic Trends 1 ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius a Li C F b Li Na K c Ge P O d C N Al e Which of the following in each pair has the larger atomic radius II Which of the following in each pair has the larger electronegativity 15 What do you notice about the answers in I vs the
More picture related to Periodic Trends Worksheet 2 Answers

Periodic Trends Worksheet Answer Key Ame my id
https://excelguider.com/wp-content/uploads/2019/07/periodic-trends-worksheet-answers-pogil-p90x-worksheets-monohybrid-as-well-as-periodic-trends-worksheet-answers-pogil.jpg
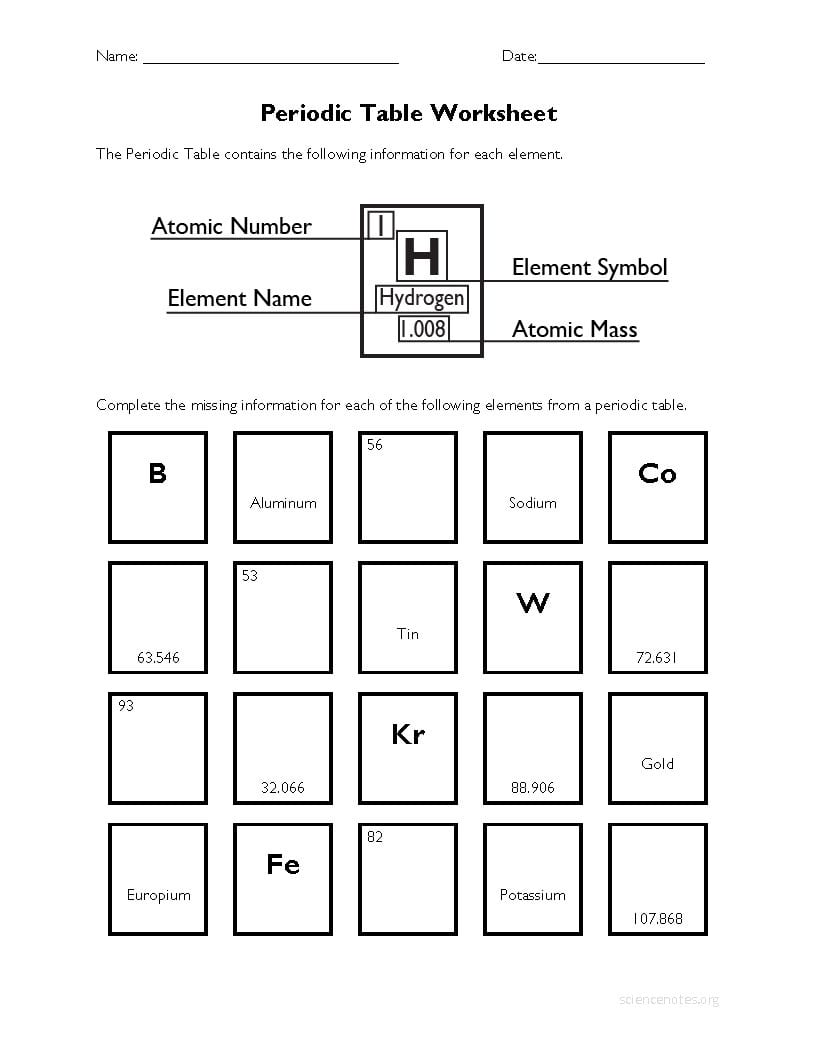
Chemistry Periodic Table Worksheet Answer Key Db excel
https://db-excel.com/wp-content/uploads/2019/09/periodic-table-worksheet-5.jpg

Trends Of The Periodic Table Worksheet Part 2 Answers Awesome Home
https://s2.studylib.net/store/data/009866394_1-45ccde6dfc0ec878ff082e205c9413ce.png
Explain why you made these choices All of the elements are in the same period The trend in atomic radius as you go across a period is DECREASING Therefore the element on the far left K is the largest and the element on the Atomic radius decreases as you go left to right across a period Potassium is in the far left group of period 4 and bromine is the farthest to the right of the four elements Explain why you made
The questions cover topics like valence electrons names and symbols of elements period and group numbers trends in properties like atomic radius ionization energy and electronegativity across periods and down groups Answer Key 3G F ext Periodic Trends Can the properties of an clement be predicted using a periodic table Why The periodic table is often considered to be the best friend of chemists

Periodic Trends Worksheet 1 Answers
https://s3.studylib.net/store/data/025283818_1-3cfb779e39f1723a8ccab497dff75852.png

Gizmo Periodic Trends Lecture Notes Bio Tech College Gizmo 2018
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/681a3baad6df1319d9a2056a3555a045/thumb_1200_1553.png
Periodic Trends Worksheet 2 Answers - Mar 13 2023 nbsp 0183 32 Know periodic trends of atomic size ionic size ionization energy and electron affinity Understand the reasons for metallic nonmetallic and metalloid character Understand