Number Of Protons And Neutrons In Hydrogen Isotopes Mass numbers of typical isotopes of Hydrogen are 1 2 The most abundant isotope hydrogen 1 protium or light hydrogen contains no neutrons and is simply a proton and an electron
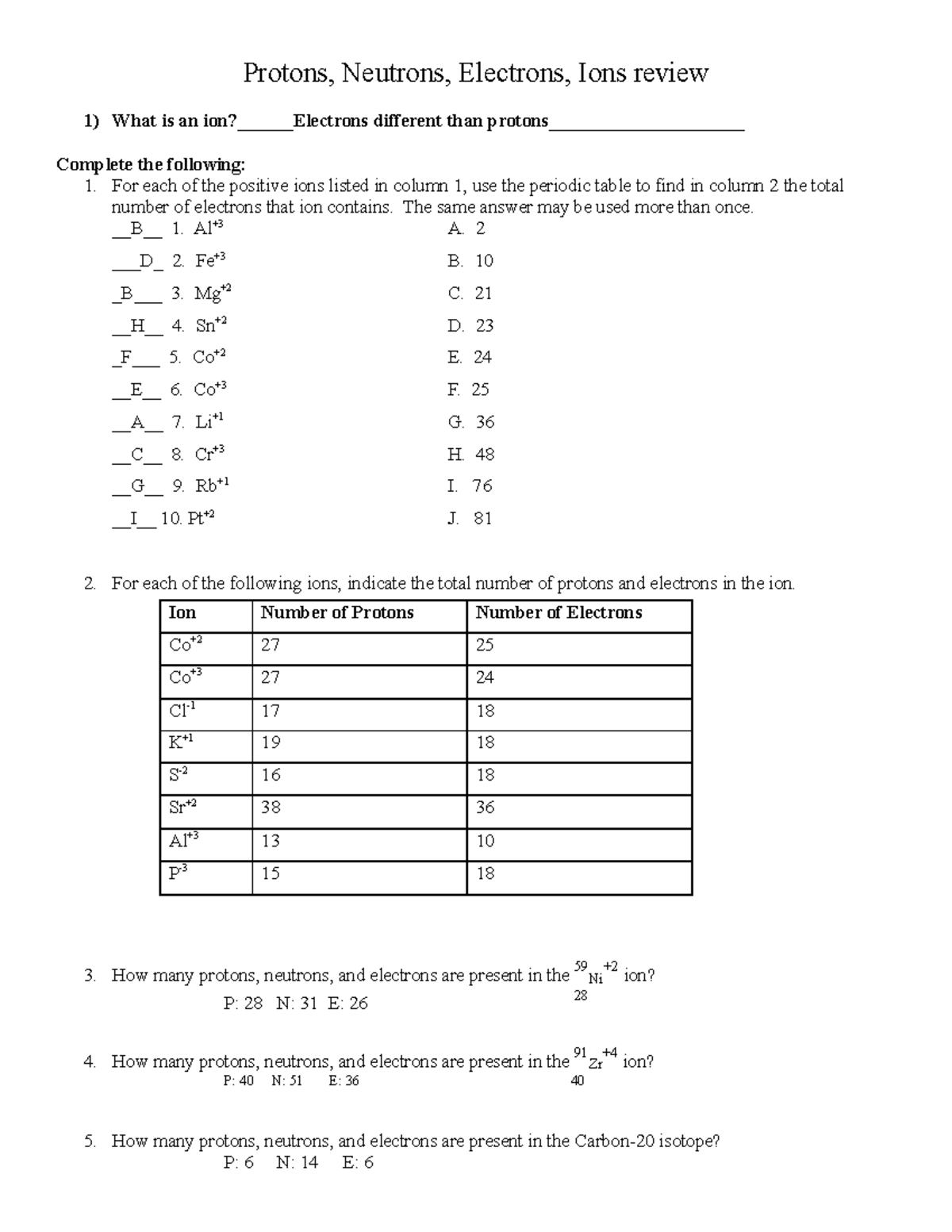
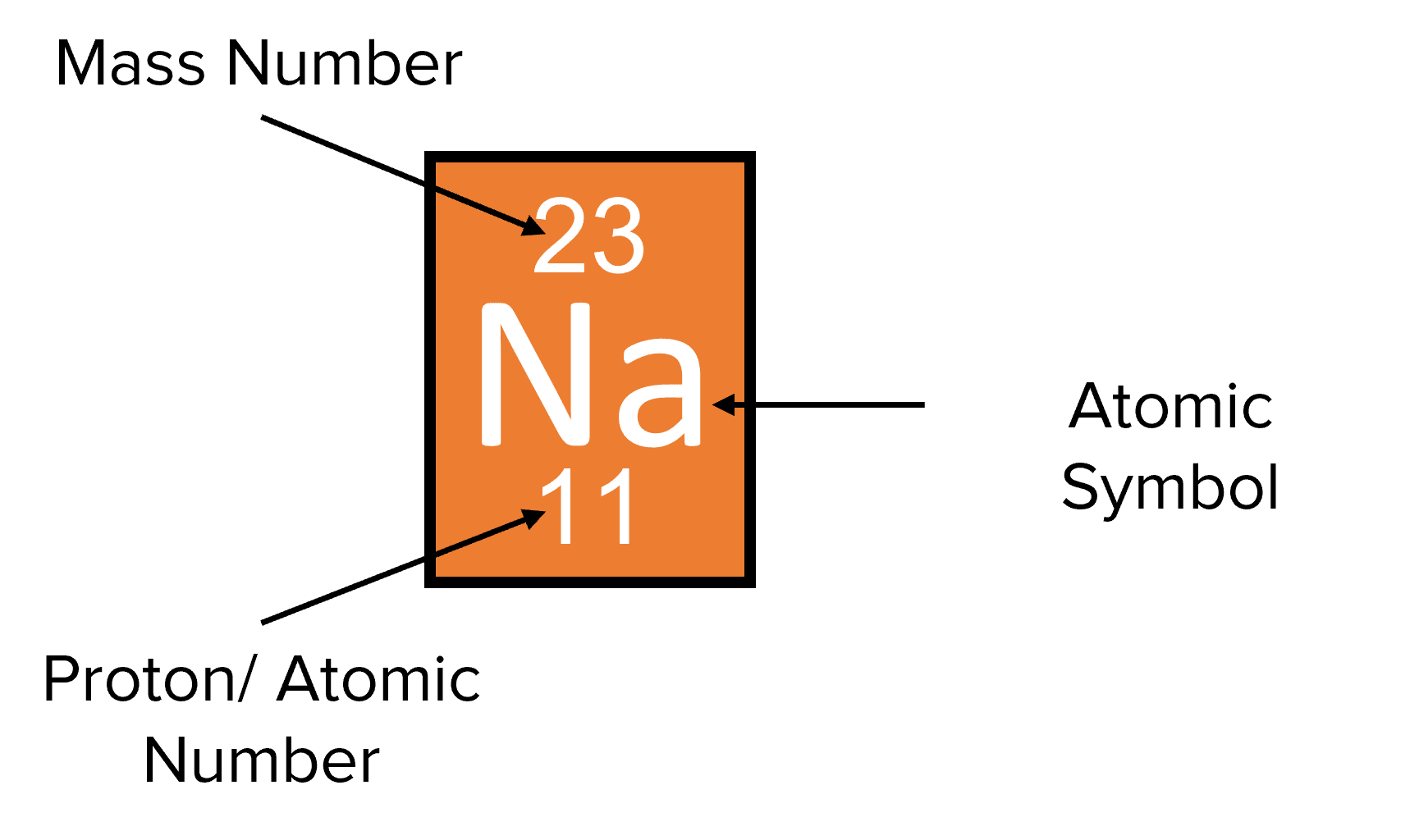
Deuterium 2 H atomic mass 2 014 101 777 844 15 Da the other stable hydrogen isotope has one proton and one neutron in its nucleus called a deuteron 2 H comprises 26 184 ppm by 1 name of the nuclide isotope 2 E isotope symbol with mass number superscript number of nucleons and Atomic number subscript number of protons 3 N number of neutrons 4
Number Of Protons And Neutrons In Hydrogen Isotopes
Number Of Protons And Neutrons In Hydrogen Isotopes
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/e26b5673f5bb007457fe46d31acf32b2/thumb_1200_1553.png

Protons Neutrons Electrons For Helium Complete Guide
https://i0.wp.com/valenceelectrons.com/wp-content/uploads/2022/06/Helium-protons-neutrons-electrons.jpg

Question Video Determining If Two Atoms Are Identical Given The Number
https://media.nagwa.com/404194847014/en/thumbnail_l.jpeg
Number of neutrons through isotopes of hydrogen The hydrogen atom has a total of seven isotopes Among the isotopes 1 H 2 H and 3 H are stable and are formed naturally The Nov 21 2023 nbsp 0183 32 Isotopes have the same number of protons but different numbers of neutrons For example the element hydrogen has three naturally occurring isotopes protium deuterium and
May 28 2024 nbsp 0183 32 The number of neutrons in the isotope can be calculated from its mass number which is written as a superscript in a nuclear symbol Mass Number of Protons of Aug 9 2000 nbsp 0183 32 There are three isotopes of the element hydrogen hydrogen deuterium and tritium How do we distinguish between them They each have one single proton Z 1 but
More picture related to Number Of Protons And Neutrons In Hydrogen Isotopes
Atoms Questions And Revision MME
https://mmerevise.co.uk/wp-content/uploads/2022/09/Na-Table-Card-1.png

The Table Gives The Numbers Of Protons Neutrons And Electrons In Some
https://us-static.z-dn.net/files/dad/461aef3e04b20dd70a8c2b985e9b00ef.png
Atomic Elements Showing The Nucleus And Shells Numbers Of Electrons
https://as1.ftcdn.net/v2/jpg/04/54/20/76/1000_F_454207645_ki3MCQPmGEAQr1AnoYWc5H1FBa2wW0sR.jpg
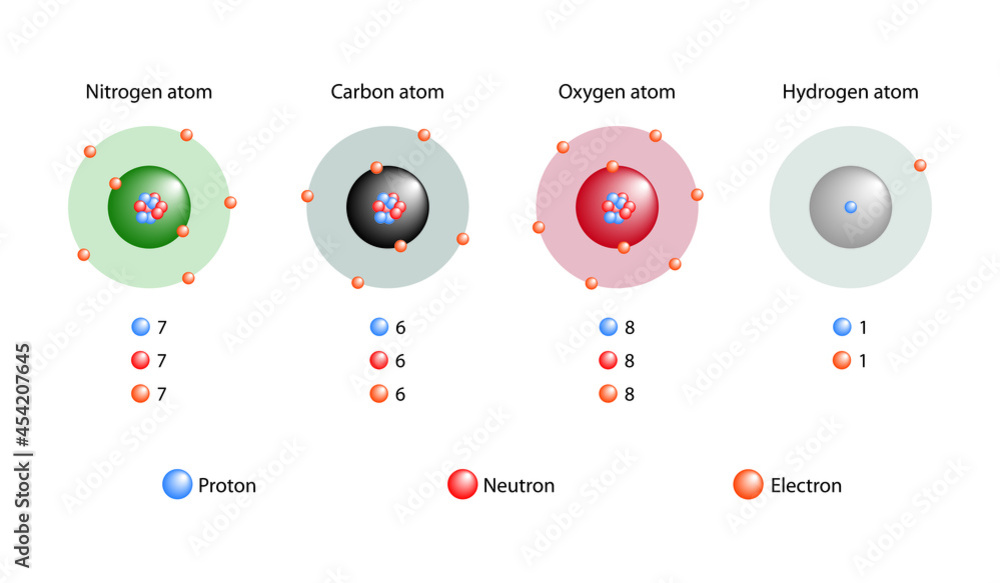
Jun 2 2023 nbsp 0183 32 An element s isotope is described as an atom with the same number of protons but a different number of neutrons The letter H is used to denote protium the normal form of Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons The number of protons in a nucleus determines the element s
Three isotopes of hydrogen are modeled in Figure 4 8 1 Most hydrogen atoms have just one proton one electron and lack a neutron These atoms are just called hydrogen Some Jan 27 2023 nbsp 0183 32 Isotopes are defined as atoms with the same number of protons and a different number of neutrons The three isotopes of hydrogen are Protium Deuterium and Tritium All
Solved Which Ion Has 45 Neutrons 36 Electrons And 35 Protons
https://www.coursehero.com/qa/attachment/33971910/

Periodic Table Element Proton Neutron Electron Periodic Table Printable
https://i0.wp.com/www.periodictableprintable.com/wp-content/uploads/2022/07/periodic-table-of-elements-list-with-protons-neutrons-and-electrons-scaled.jpg?resize=1536%2C1164&ssl=1
Number Of Protons And Neutrons In Hydrogen Isotopes - 2 H the other stable hydrogen isotope is known as deuterium and contains one proton and one neutron in its nucleus Deuterium comprises 0 0026 0 0184 by mole fraction or atom