Name Three Factors That Affect The Rate Of Chemical Reactions The factors that affect the rate of a chemical reaction are as follows Nature of the reactants Nature or reactivity of reactants influences the rate of a chemical reaction E g Al is more reactive than Zn Therefore the rate of reaction of Al with hydrochloric acid is higher than that of Zn
Reactant concentration the physical state of the reactants and surface area temperature and the presence of a catalyst are the four main factors that affect reaction rate The factors that affect the rate of a reaction are as follows i Nature of the reactant The rate of reaction depends on the nature of the reactant For instance ionic molecules react more quickly than covalent ones ii State of reactants Solid reactions are slower liquid reactions are faster and gas reactions are very quick
Name Three Factors That Affect The Rate Of Chemical Reactions

Name Three Factors That Affect The Rate Of Chemical Reactions
https://i.ytimg.com/vi/wqMswod1K0Y/maxresdefault.jpg

Rate Of Dissolving And Factors That Affect It YouTube
https://i.ytimg.com/vi/0SQySg9ShGU/maxresdefault.jpg
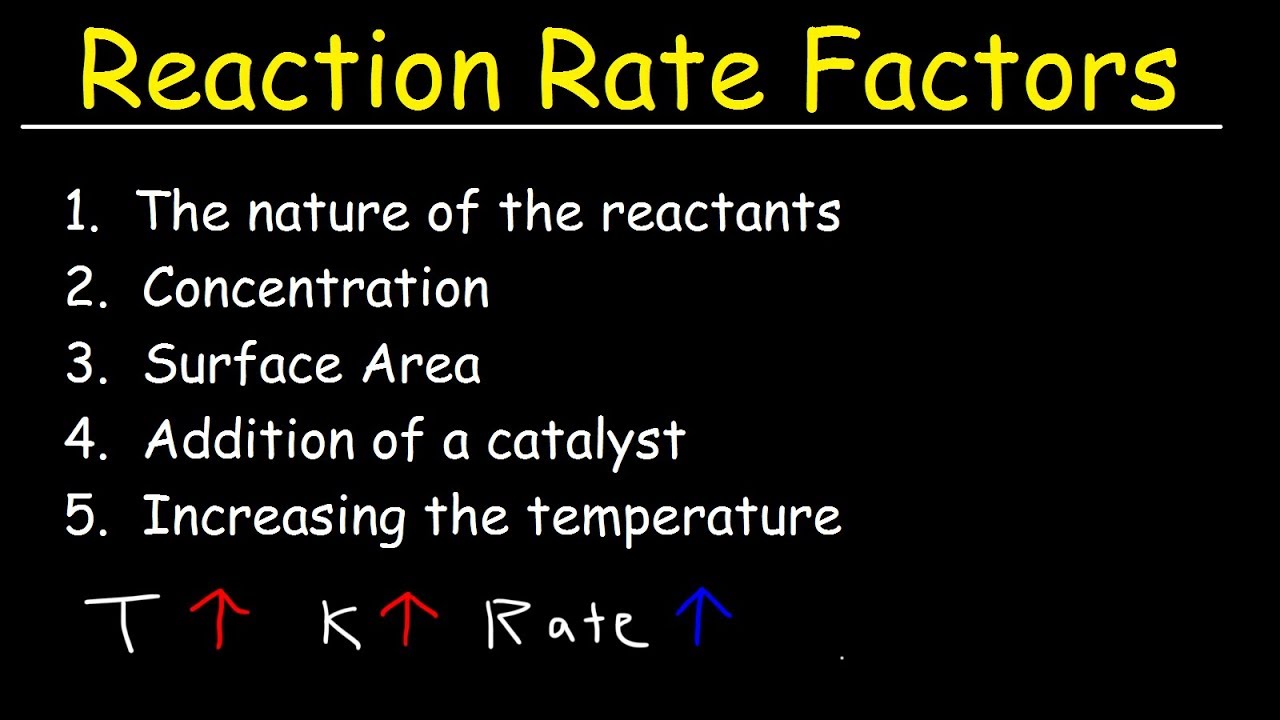
Factors Affecting The Rate Of The Reaction Chemical Kinetics YouTube
https://i.ytimg.com/vi/JpoOfrPKgmM/maxresdefault.jpg
FACTORS AFFECTING RATE OF CHEMICAL REACTIONS The rate of a chemical reaction is affected by several factors like 1 Concentration of reactants 2 Pressure 3 Temperature 4 Catalyst 5 Nature of reactants 6 Orientation of reacting species 7 Surface area 8 Intensity of light 9 Nature of solvent The effect of these factors are Jun 19 2020 nbsp 0183 32 We can identify five factors that affect the rates of chemical reactions the chemical nature of the reacting substances the state of subdivision one large lump versus many small particles of the reactants the temperature of the reactants the concentration of the reactants and the presence of a catalyst
There are four factors that affect the rate speed of a chemical reaction catalyst Catalysts are substances that speed up chemical reactions but can be recovered chemically unchanged at the Some chemical reactions are nearly instantaneous while others usually take some time to reach the final equilibrium This article aims to help students learn about and understand what exactly is the rate of reaction for a given chemical compound
More picture related to Name Three Factors That Affect The Rate Of Chemical Reactions

GCSE Chemistry Factors Affecting The Rate Of Reaction 47 YouTube
https://i.ytimg.com/vi/-4HXaUBbv04/maxresdefault.jpg
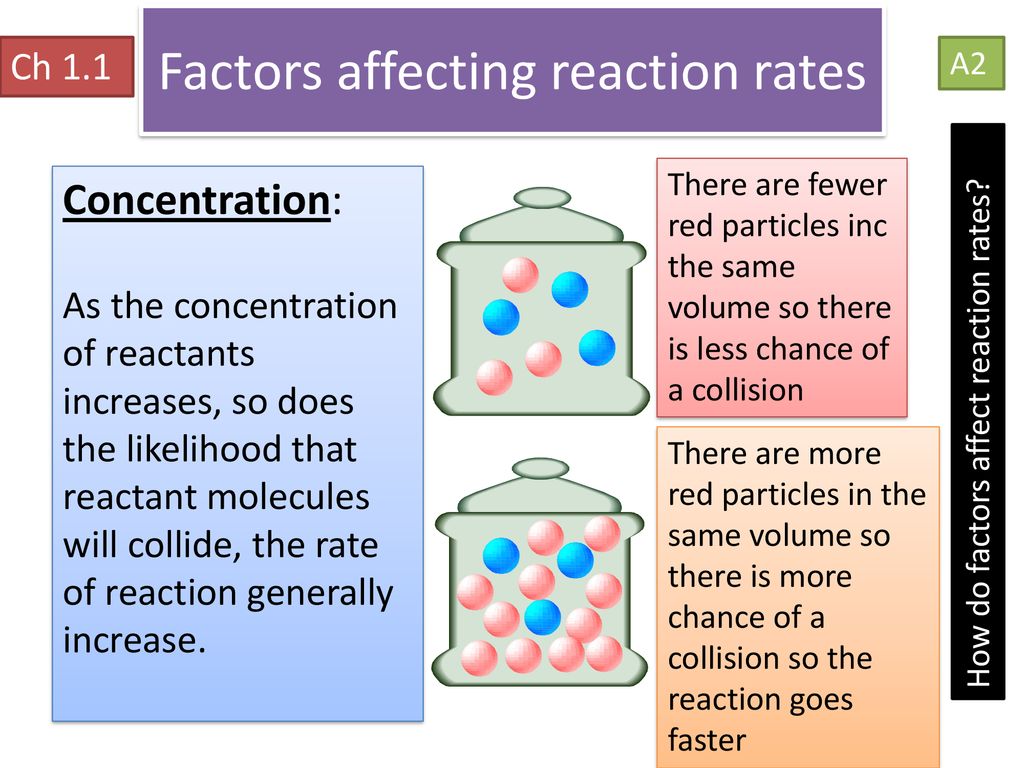
Chapter 1 Rate Of Reaction Ppt Download
https://slideplayer.com/slide/15098838/91/images/6/Factors+affecting+reaction+rates.jpg

Factors That Affect Diffusion Diagram Quizlet
https://o.quizlet.com/y8hnr1Iai4N.iZXeupAdpA_b.png
This information is obtained by studying the chemical kinetics of a reaction which depend on various factors reactant concentrations temperature physical states and surface areas of reactants and solvent and catalyst properties if either are present Nov 26 2019 nbsp 0183 32 Several factors can influence the chemical reaction rate In general a factor that increases the number of collisions between particles will increase the reaction rate and a factor that decreases the number of collisions between particles will
Reactions occur when two reactant molecules effectively collide each having minimum energy and correct orientation Reactant concentration the physical state of the reactants and surface area temperature and the presence of a catalyst are There are four main factors that can affect the reaction rate of a chemical reaction 1 Reactant concentration Increasing the concentration of one or more reactants will often increase the rate of reaction This occurs because a higher concentration of a reactant will lead to more collisions of that reactant in a specific time period 2

Question Video Determining The Factor That Does Not Affect The Rate Of
https://media.nagwa.com/480109852128/en/thumbnail_l.jpeg

Question Video Selecting A Factor That Does Not Affect Reaction Rate
https://media.nagwa.com/268169073214/en/thumbnail_l.jpeg
Name Three Factors That Affect The Rate Of Chemical Reactions - May 25 2021 nbsp 0183 32 The rate of reaction was discussed in terms of three factors collision frequency the collision energy and the geometric orientation Remember that the collision frequency is the number of collisions per second