Mole And Avogadro S Number Aug 10 2022 nbsp 0183 32 Use Avogadro s number to convert to moles and vice versa given the number of particles of an element Know the definition of the mole Determine the formula mass of an ionic or molecular compound Determine the percent composition of each element in a compound from the chemical formula
Definition of the Mole and Avogadro s Number The mole or mol is a unit of measurement in chemistry used to designate a very large number of molecules atoms or particles This very large number is called Avogadro s Number 6 02214 x 10 23 the number of units in a mole These numbers are very important for telling us about the Define the amount unit mole and the related quantity Avogadro s number Calculate molar mass of a compound from the molecular formula
Mole And Avogadro S Number
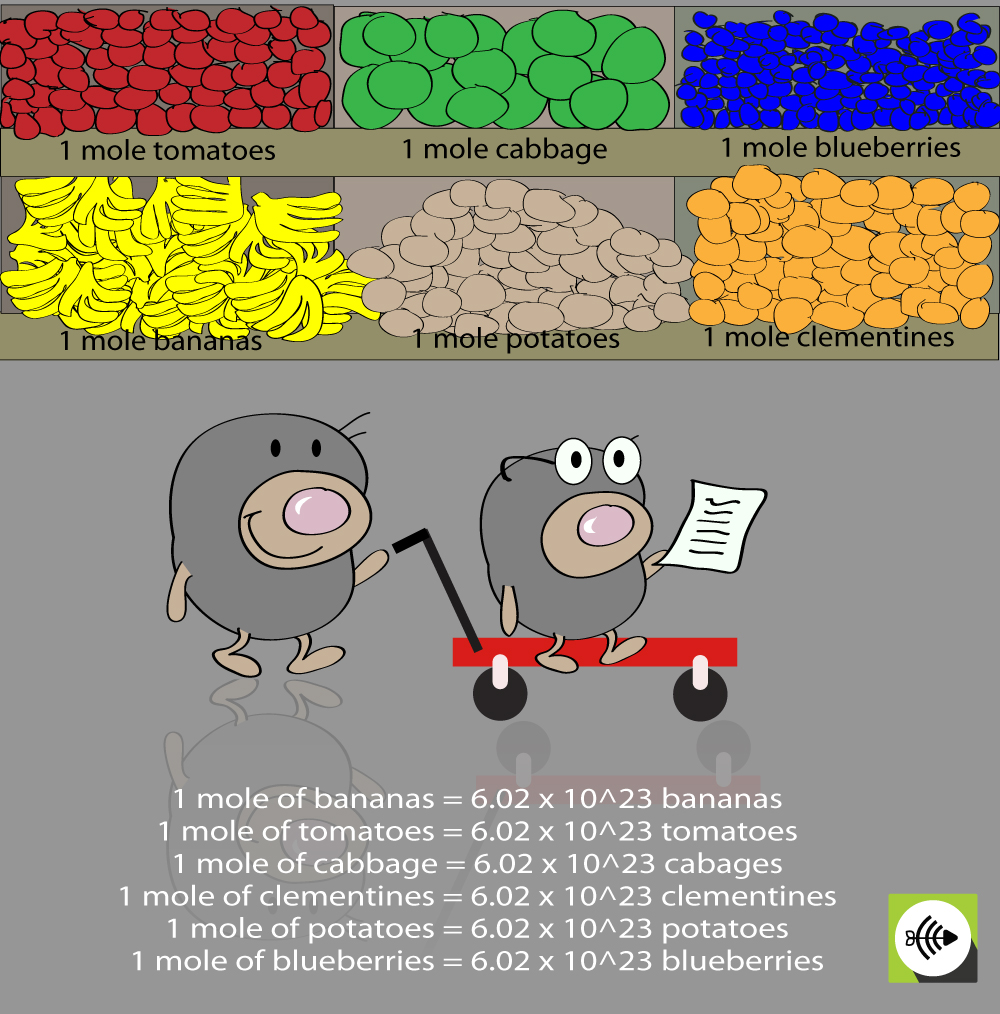
Mole And Avogadro S Number
http://surfguppy.com/wp-content/uploads/mole-shopping.jpg

AS Chemistry The Mole And The Avogadro Constant Teaching Resources
https://d1e4pidl3fu268.cloudfront.net/785c29fa-360f-4813-8a98-f2281652f9f9/Screenshot20191013at192525.png
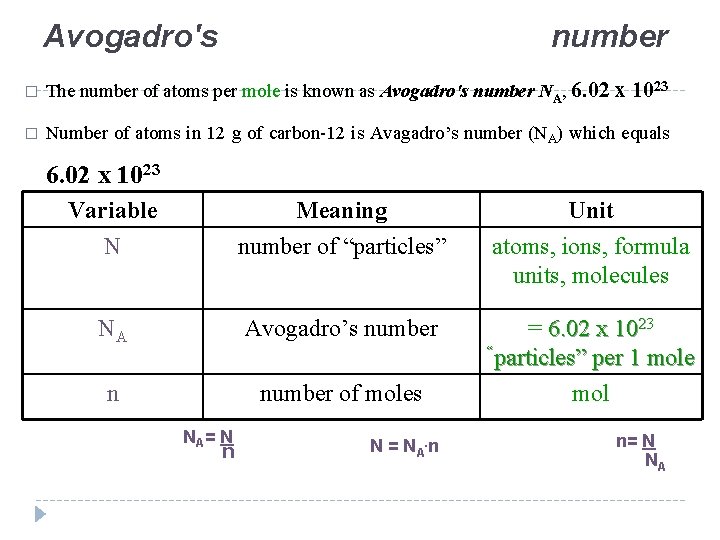
Lecture 5 THE MOLE Avogadros Number The Mole
https://slidetodoc.com/presentation_image_h/d12b552967ebbc7c2a625c2df4141adf/image-4.jpg
Sep 26 2024 nbsp 0183 32 Here s an explanation of what these terms mean how they relate to Avogadro s number how to use them to find molecular and formula weight and how to convert molecules to moles A molecule is a combination of two or more atoms that are held together by chemical bonds such as covalent bonds and ionic bonds Sep 25 2024 nbsp 0183 32 Understand the mole and Avogadro Constant for IGCSE Chemistry Learn how to calculate moles molar mass and molar gas volumes with ease
The number of entities composing a mole has been experimentally determined to be 6 02214179 215 10 23 a fundamental constant named Avogadro s number N A or the Avogadro constant in honor of Italian scientist Amedeo Avogadro Jan 26 2021 nbsp 0183 32 Avogadro s number is the number of units of any substance in one mole It is also called Avogadro s constant Despite the name Amedeo Avogadro did not discover or describe Avogadro s number Instead it s named in honor of Avogadro s contributions to the
More picture related to Mole And Avogadro S Number

Avogadro s Constant Surfguppy Chemistry Made Easy Visual Learning
http://surfguppy.com/wp-content/uploads/mole-forehead-1000x1070.jpg
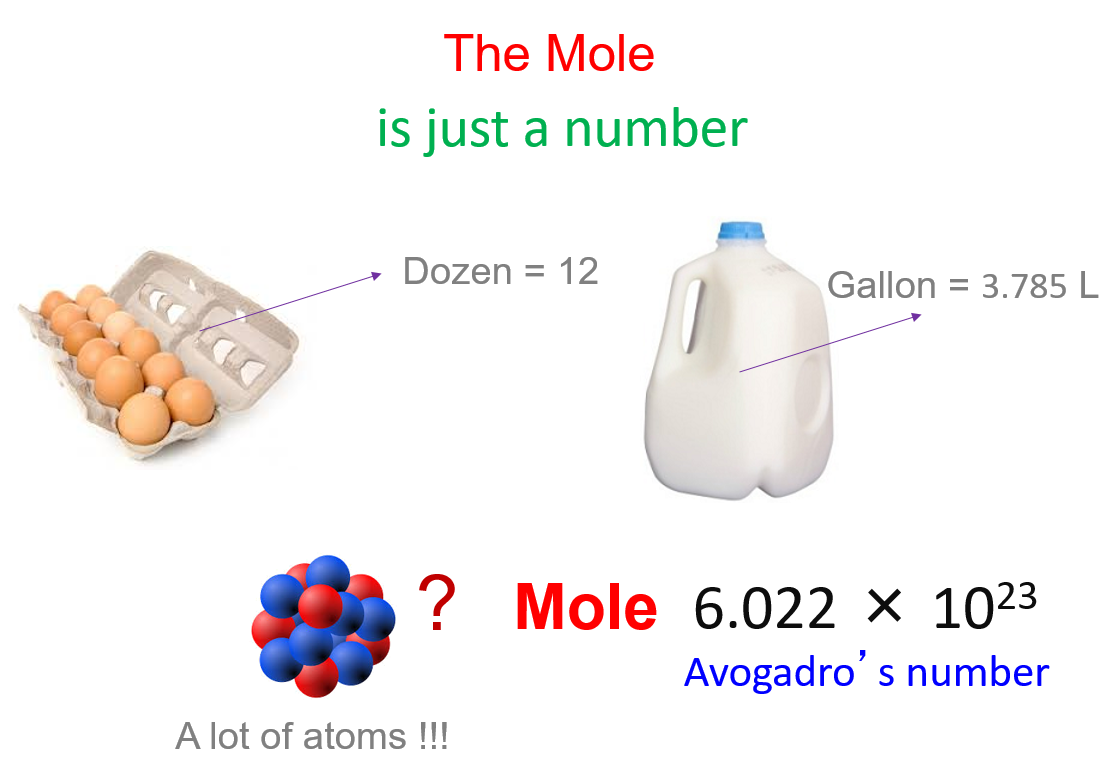
The Mole And Molar Mass Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/04/mole-avogadros-number.png
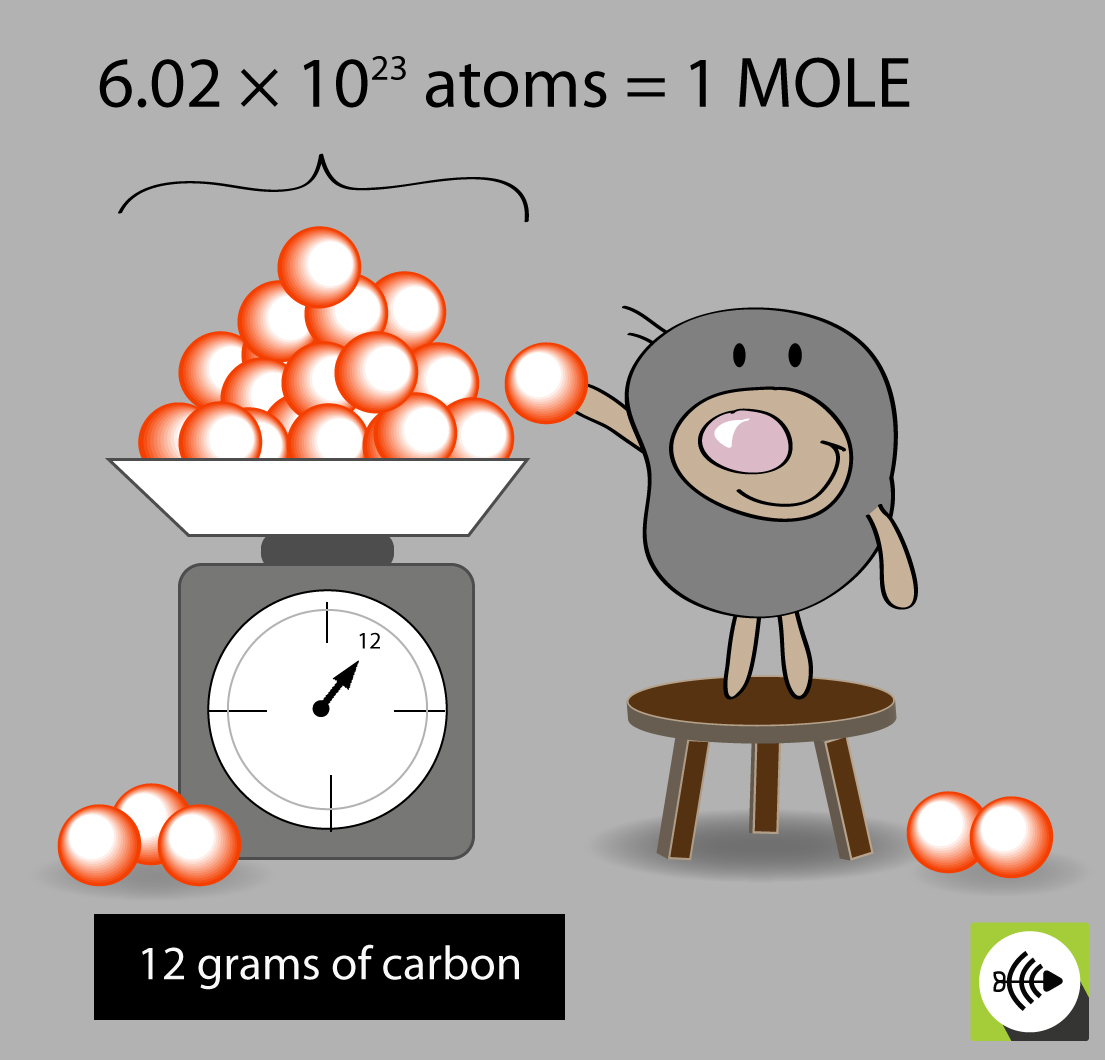
Avogadro s Constant Surfguppy Chemistry Made Easy Visual Learning
http://surfguppy.com/wp-content/uploads/MOLES-carbon.jpg
What is the mole and Avogadro s constant The mole is the unit for amount of substance The number of particles in a substance can be found using the Avogadro constant The mass of Avogadro s number is the link between the number of atoms or molecules or ions of a material and the number of moles of material Understanding it allows chemical equations to be represented through the ratios of compounds and particles enabling us to upscale reactions outside of individual atoms to notable masses and quantities for
[desc-10] [desc-11]
Avogadro s Constant Surfguppy Chemistry Made Easy For Visual Learners
https://surfguppy.com/wp-content/uploads/mole-number-atoms-bananas-1000x936.jpg

Moles And Avogadro s Number
https://i.ytimg.com/vi/TCLrVxgk7Dg/maxresdefault.jpg
Mole And Avogadro S Number - The number of entities composing a mole has been experimentally determined to be 6 02214179 215 10 23 a fundamental constant named Avogadro s number N A or the Avogadro constant in honor of Italian scientist Amedeo Avogadro