Mhra Referencing Footnote Example Apr 16 2019 nbsp 0183 32 I m writing some guidance on equipment management and fridge temperature settings and alarm points seems to be an issue across pathology and I would appreciate some
Aug 1 2023 nbsp 0183 32 The 21st edition of the Guide to the preparation use and quality assurance of blood components providing state of the art guidance for healthcare professionals has just been Apr 23 2020 nbsp 0183 32 This forum should not be used for direct questions to the GCP Inspectorate Clinical Trials Unit reporting serious breaches or making formal complaints These should follow the
Mhra Referencing Footnote Example

Mhra Referencing Footnote Example
https://i.ytimg.com/vi/P0O-1fMIsIE/maxresdefault.jpg

6 Footnotes And Oxford Referencing Style Footnote Referencing YouTube
https://i.ytimg.com/vi/jtP_vq15sVY/maxresdefault.jpg

Notes And Bibliography Footnotes Chicago Referencing Style Guides
https://libapps-au.s3-ap-southeast-2.amazonaws.com/accounts/176225/images/Chicago_footnotes_example_text.jpg
2025 Blood Compliance Report Hospital Blood Bank Compliance Report The compliance report and declaration forms for Hospital Blood Banks HBB 01st April 2024 to 31st March 2025 are Mar 2 2013 nbsp 0183 32 I cant imagine that a licenesed drug would be given out with no expiry so why should a trial drug MHRA moderator perhaps this can be fed back to the next consultation
Aug 1 2015 nbsp 0183 32 The MHRA GCP Guide 2012 14 2 Quality Systems states demonstrate that training has occurred documentation must2 be maintained and retained for all staff The Aug 7 2018 nbsp 0183 32 Forum Good Clinical Practice GCP Regulatory documents including protocol clinical study reports and publications Electronic signatures which type are acceptable
More picture related to Mhra Referencing Footnote Example

Naaheavy Blog
https://libapps-au.s3-ap-southeast-2.amazonaws.com/accounts/176225/images/super_no_and_footnotes.png
+-+a+numerical+style..jpg)
Headington School Oxford Ppt Download
https://slideplayer.com/slide/16158842/95/images/18/Referencing+Skills+Modern+Humanities+Referencing+Association+(MHRA)+-+a+numerical+style..jpg
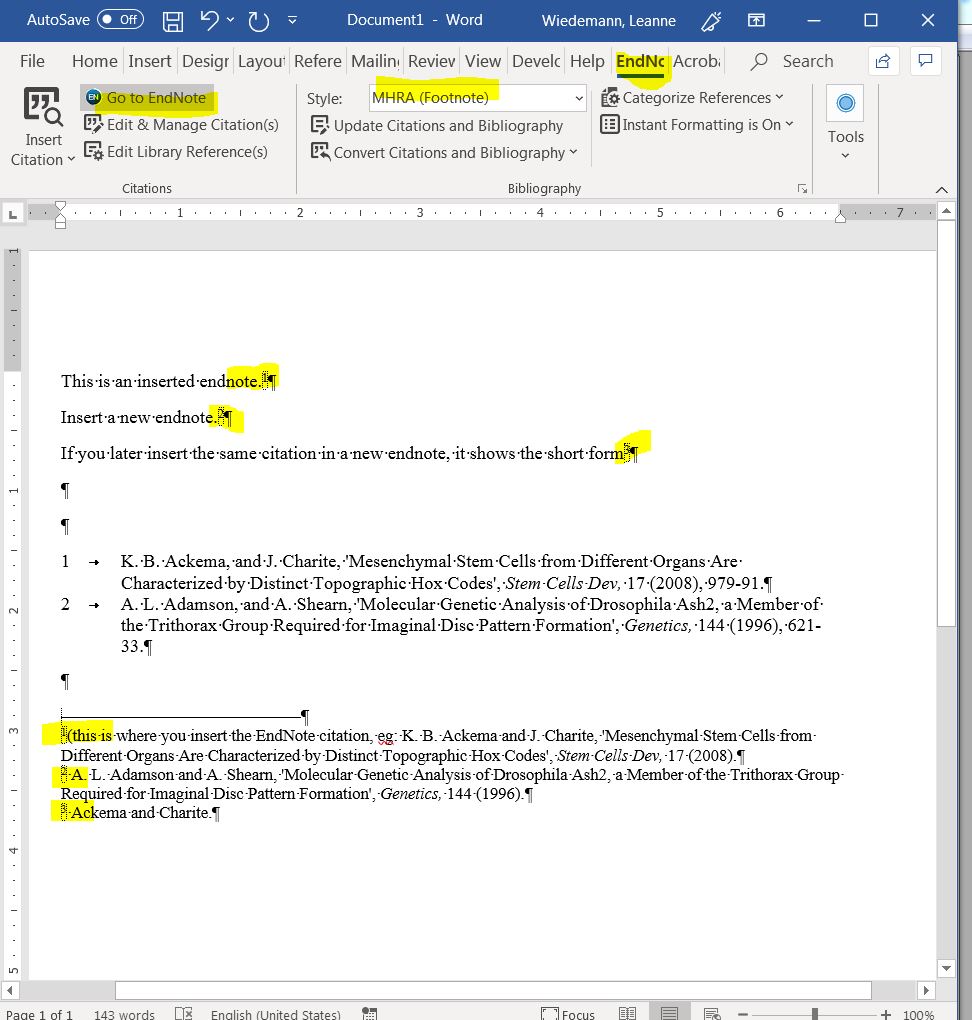
MHRA Style Footnotes Short Form EndNote How To Discourse
https://europe1.discourse-cdn.com/endnote/original/2X/9/9dc8505ba294a9b0700e0e71c8ba03c8b7ab1881.jpeg
Jun 1 2016 nbsp 0183 32 I got this reply from MHRA Regarding your query yes an IMP manufacturedwithin the EU needs to be QP certified even if it is going to be used inclinical trials in territories Aug 5 2021 nbsp 0183 32 Forum Good Clinical Practice GCP Investigator Sites inc Principal Investigator responsibilities consent source data and CRFs Sub Co Investigator absence and delegation
[desc-10] [desc-11]

Scaling Solar A World Bank Group Solution To Rapidly Expand Private
https://slideplayer.com/slide/11959602/68/images/5/The+WBG+solution:+A+One-Stop-Shop+offering.jpg

Oscola Referencing Guide Vrogue co
https://libapps-au.s3-ap-southeast-2.amazonaws.com/accounts/206811/images/Footnote_sample_1.png
Mhra Referencing Footnote Example - Mar 2 2013 nbsp 0183 32 I cant imagine that a licenesed drug would be given out with no expiry so why should a trial drug MHRA moderator perhaps this can be fed back to the next consultation