Mass To Mass Stoichiometry Worksheet Chemistry Stoichiometry Problem Sheet 1 Directions Solve each of the following problems Show your work including proper units to earn full credit 1 Silver and nitric acid react according to the following balanced equation 3 Ag s 4 HNO 3 aq 3 AgNO 3 aq 2 H 2 O l NO g A
A What mass of iron is needed to react with 16 0 grams of sulfur 27 87 g Fe b How many grams of FeS are produced 43 9 g FeS We can use that relation to answer stoichiometry questions in terms of the masses of a particular substance in addition to moles We do this using the following sequence moles of A molar ratio moles of B molar mass grams of B Collectively these conversions are called mole to mass calculations
Mass To Mass Stoichiometry Worksheet
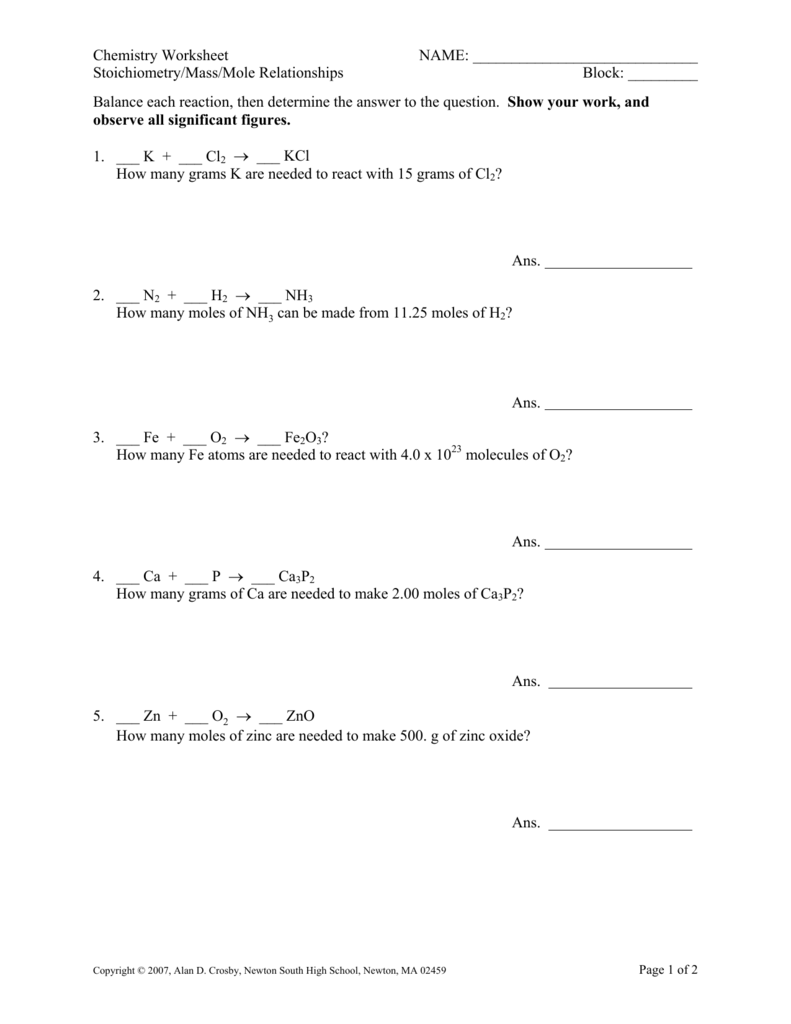
Mass To Mass Stoichiometry Worksheet
https://s3.studylib.net/store/data/008865292_1-66353eb3924ad145d822942b625669e4.png

Reaction Stoichiometry Chem 10 Review Worksheet
https://i.pinimg.com/736x/93/09/86/93098610056723383c7943bb3138fad2.jpg

Mass To Mass Stoichiometry Worksheet Walkthrough YouTube
https://i.ytimg.com/vi/3SLbcaYBb8w/maxresdefault.jpg
Sep 21 2022 nbsp 0183 32 Mass mass calculations involve converting the mass of a reactant to moles of reactant then using mole ratios to determine moles of product which can then be converted to mass of product Stoichiometry Mass Mass Examples This is the most common type of stoichiometric problem in high school There are four steps involved in solving these problems Make sure you are working with a properly balanced chemical equation Convert grams
Mar 13 2023 nbsp 0183 32 Convert between numbers of atoms moles and mass of sample by using Avogadro s number and the appropriate molar mass Calculate the empirical formula of a compound from percent composition data Calculate mass relationships between reactants and products based on a balanced chemical equation Mass Mass Stoichiometry Worksheet Remember to balance each equation if it is not already before solving the following stoichiometry problems Show your work 1 Determine the mass of lithium hydroxide produced when 0 g of lithium nitride reacts with water according to the following equation Li 3 N 3H 2 O NH 3 3LiOH B C A 2
More picture related to Mass To Mass Stoichiometry Worksheet
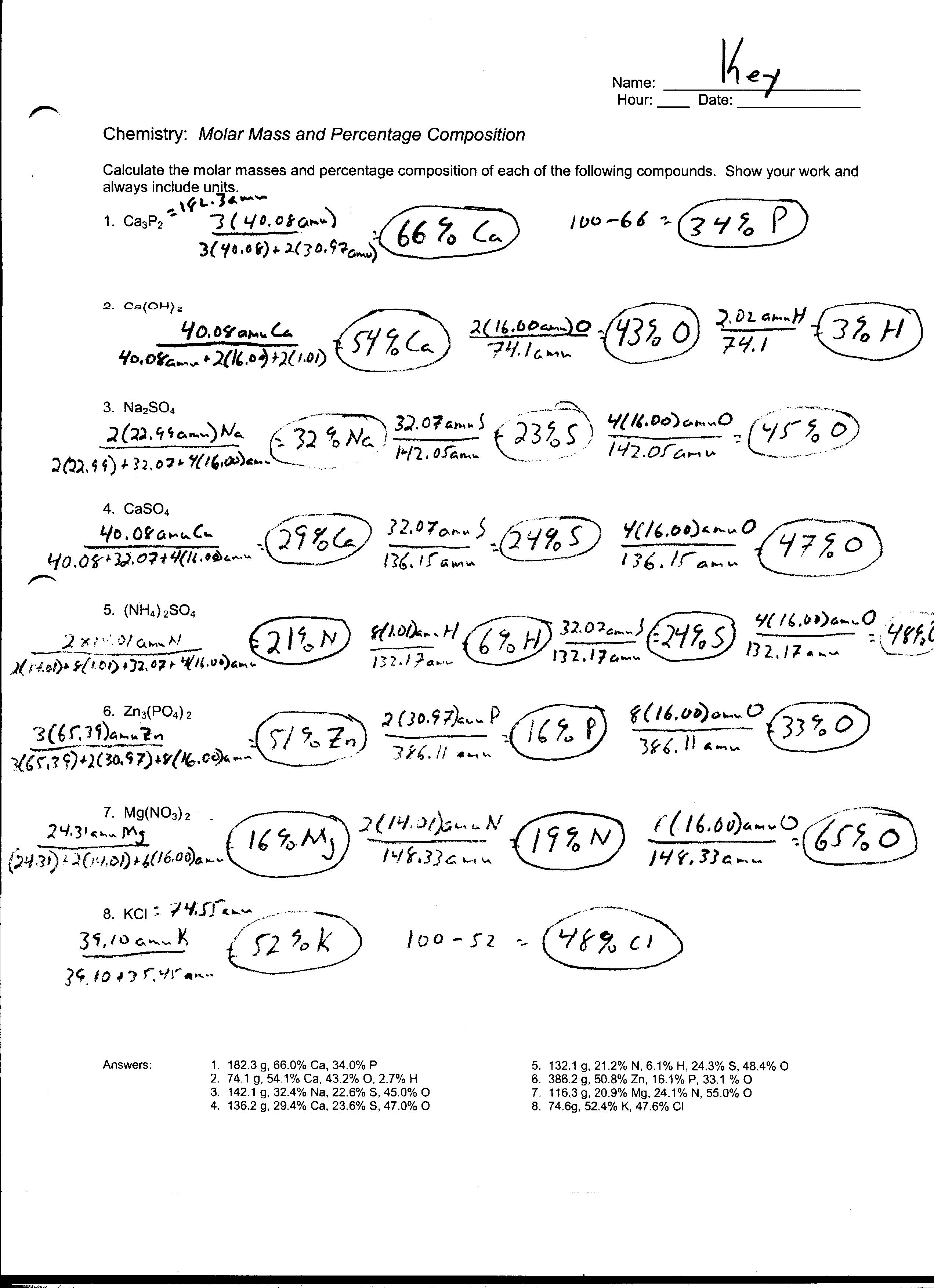
5 Best Images Of Chemistry If8766 Worksheet Answers Mass To Mole
http://www.worksheeto.com/postpic/2013/06/mass-to-mole-stoichiometry-worksheet-answer-key_708283.jpg

Mass Volume Problems
https://s3.studylib.net/store/data/008595049_1-cecb09782cce4ec52993c15878e4ceb2.png

Stoichiometry Mass To Mass Practice 3 YouTube
https://i.ytimg.com/vi/WRA7-aKs3Yk/maxresdefault.jpg
Oct 1 2024 nbsp 0183 32 Mass mass calculations are the most practical of all mass based stoichiometry problems Moles cannot be measured directly while the mass of any substance can generally be easily measured in the lab Stoichiometry Mass to Mass Conversions Wksht 1 Converting mass to mass Click LT 1 Mass Mass Conversions wkst 1 doc link to view the file Video Tutorial on Stoichiometry from Khan Academy
Mass to Mass Stoichiometry Problems In the following problems calculate how much of the indicated product is made Show all your work 1 LiOH HBr LiBr H2O If you start with 10 0 grams of lithium hydroxide how many grams of lithium bromide will be produced 2 C2H4 3 O2 2 CO2 2 H2O Mass to Mass Stoichiometry Problems Answer Key In the following problems calculate how much of the indicated product is made Show all your work If you start with ten grams of lithium hydroxide how many grams of lithium bromide will be produced If you start with 45 grams of ethylene C2H4 how many grams of carbon dioxide will be

Quiz Worksheet Mass to Mass Stoichiometric Calculations Study
https://study.com/academy/practice/quiz-worksheet-mass-to-mass-stoichiometric-calculations.jpg
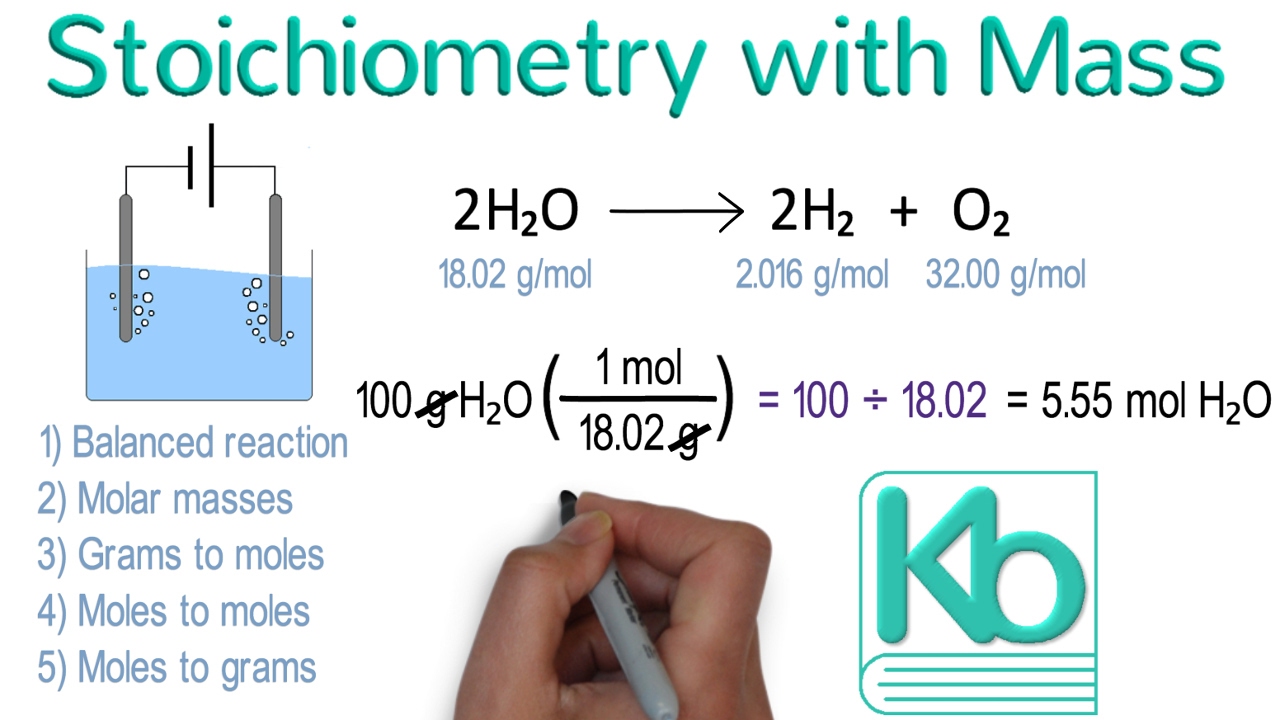
Stoichiometry With Mass Stoichiometry Tutorial Part 2 YouTube
https://i.ytimg.com/vi/BZuS3Agn4pI/maxresdefault.jpg
Mass To Mass Stoichiometry Worksheet - Mass Mass Stoichiometry Worksheet Remember to balance each equation if it is not already before solving the following stoichiometry problems Show your work 1 Determine the mass of lithium hydroxide produced when 0 g of lithium nitride reacts with water according to the following equation Li 3 N 3H 2 O NH 3 3LiOH B C A 2