Mass To Mass Conversion Stoichiometry Enter the chemical equation and the calculator will readily calculate the number of reactants and products involved in it This stoichiometry calculator lets you calculate the relative amounts of reactants and products involved in a chemical reaction
Nov 21 2023 nbsp 0183 32 How do you convert mass to mass in stoichiometry To convert from mass of substance A to mass of substance B in a chemical equation first convert the mass of substance A to Convert the mass of one substance substance A to the corresponding number of moles using its molar mass From the balanced chemical equation obtain the number of moles of another substance B from the number of moles of substance A using the appropriate mole ratio the ratio of their coefficients
Mass To Mass Conversion Stoichiometry
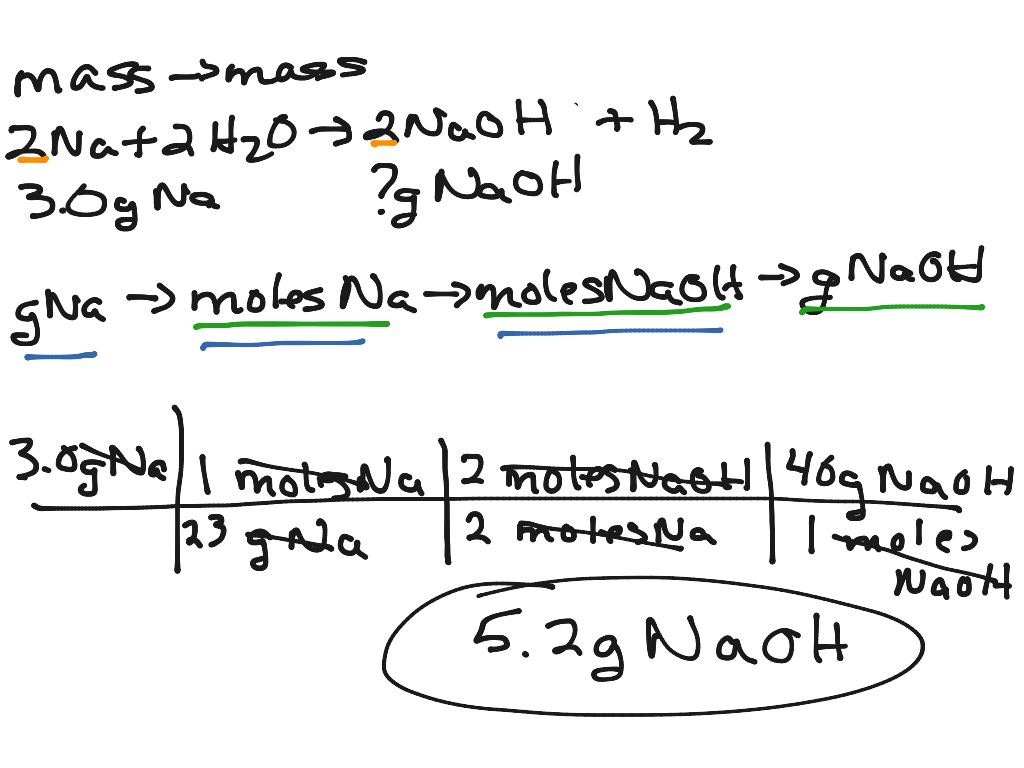
Mass To Mass Conversion Stoichiometry
https://showme0-9071.kxcdn.com/files/650639/pictures/thumbs/1529284/last_thumb1398816077.jpg
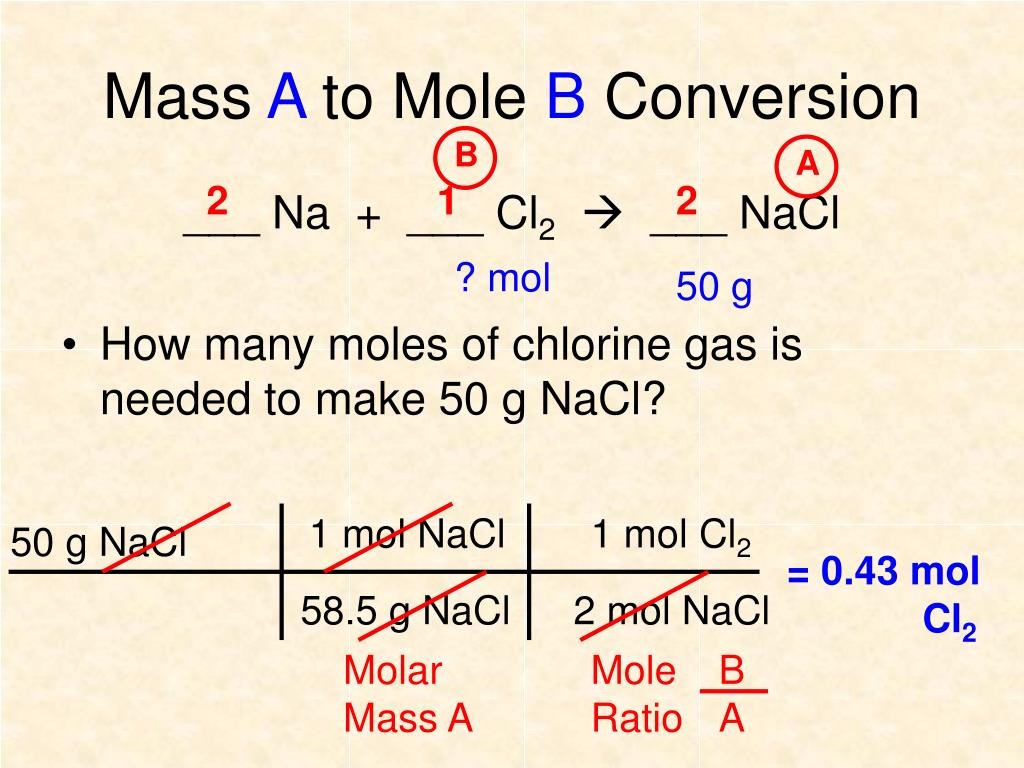
PPT Chapter 12 Stoichiometry PowerPoint Presentation Free Download
https://image2.slideserve.com/4011195/mass-a-to-mole-b-conversion1-l.jpg
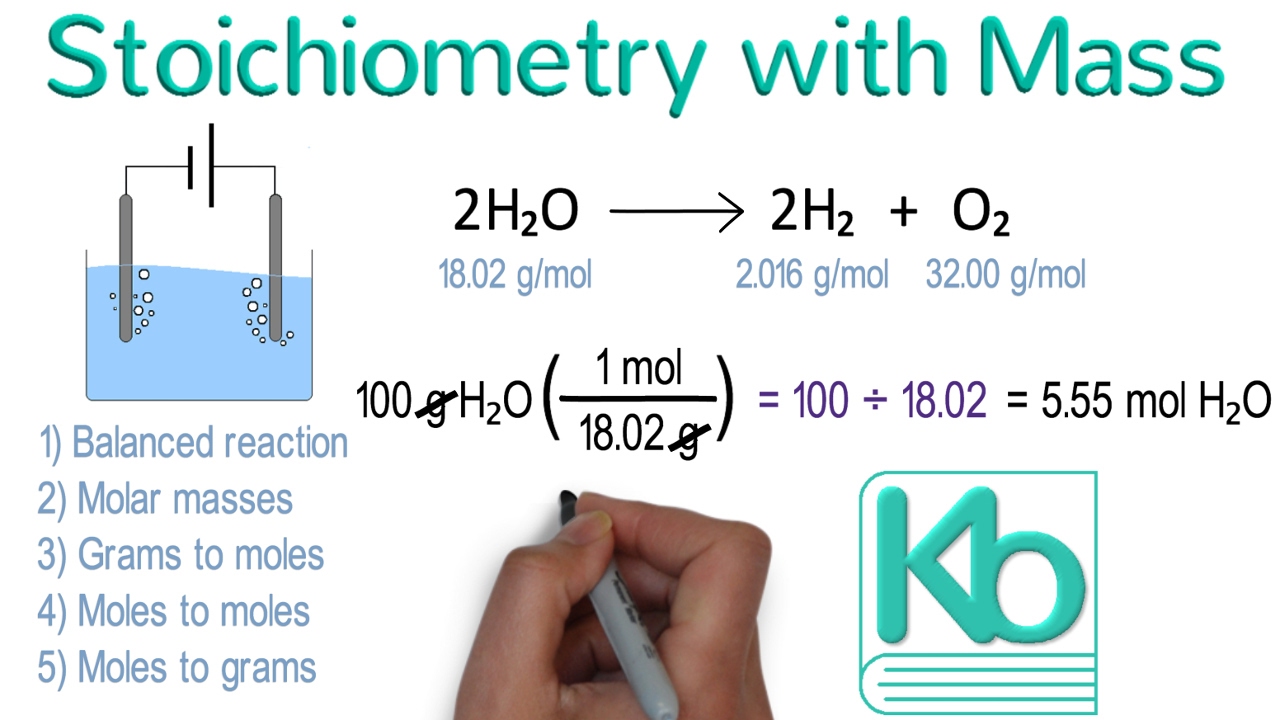
Stoichiometry With Mass Stoichiometry Tutorial Part 2 YouTube
https://i.ytimg.com/vi/BZuS3Agn4pI/maxresdefault.jpg
Dec 5 2024 nbsp 0183 32 Mass mass calculations are the most practical of all mass based stoichiometry problems Moles cannot be measured directly while the mass of any substance can generally be easily measured in the lab This type of problem is three Our solutions demonstrate how to use the factor label method conversion factors with an understanding of mole ratios and molar mass to solve for the unknown The problems on this page are three step problems
Jan 15 2024 nbsp 0183 32 Recall that we can relate a molar amount to a mass amount using molar mass We can use that ability to answer stoichiometry questions in terms of the masses of a particular substance in addition to moles We do this using the following sequence Collectively these conversions are called mole mass calculations To convert from one mass substance A to another mass substance B you must convert the mass of A first to moles then use the mole to mole conversion factor B A then convert the mole amount of B back to grams of B
More picture related to Mass To Mass Conversion Stoichiometry

Stoichiometry Mass To Mass Practice 3 YouTube
https://i.ytimg.com/vi/WRA7-aKs3Yk/maxresdefault.jpg

SimplyChemistry C3 MOLE MASS NO PARTICLE CONVERSIONS
https://1.bp.blogspot.com/-l3YrK-LcQSA/VYc6rIdwJ7I/AAAAAAAABqI/2LRCdU4gi3c/s1600/SAEU_01.jpg

Stoichiometry Mass To Mass Practice 1 YouTube
https://i.ytimg.com/vi/0KZ7CFbe98g/maxresdefault.jpg
A stoichiometric diagram of the combustion reaction of methane Stoichiometry s t k i m t r i is the relationships between the masses of reactants and products before during and following chemical reactions Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the Molar mass allows us to convert the mass of a substance present to the number of moles using the atomic weight of elements from the periodic table Molar mass of compounds is determined through adding the molar masses of individual elements
Jul 30 2023 nbsp 0183 32 Mass to mass calculation is a type of stoichiometry problem where you convert the mass of one substance reactant to the mass of another substance product using the balanced chemical equation and stoichiometric ratios There are four steps involved in solving these problems Make sure you are working with a properly balanced chemical equation Convert grams of the substance given in the problem to moles Construct two ratios one from the problem and one from the chemical equation and set them equal The ratio from the problem will have an unknown x
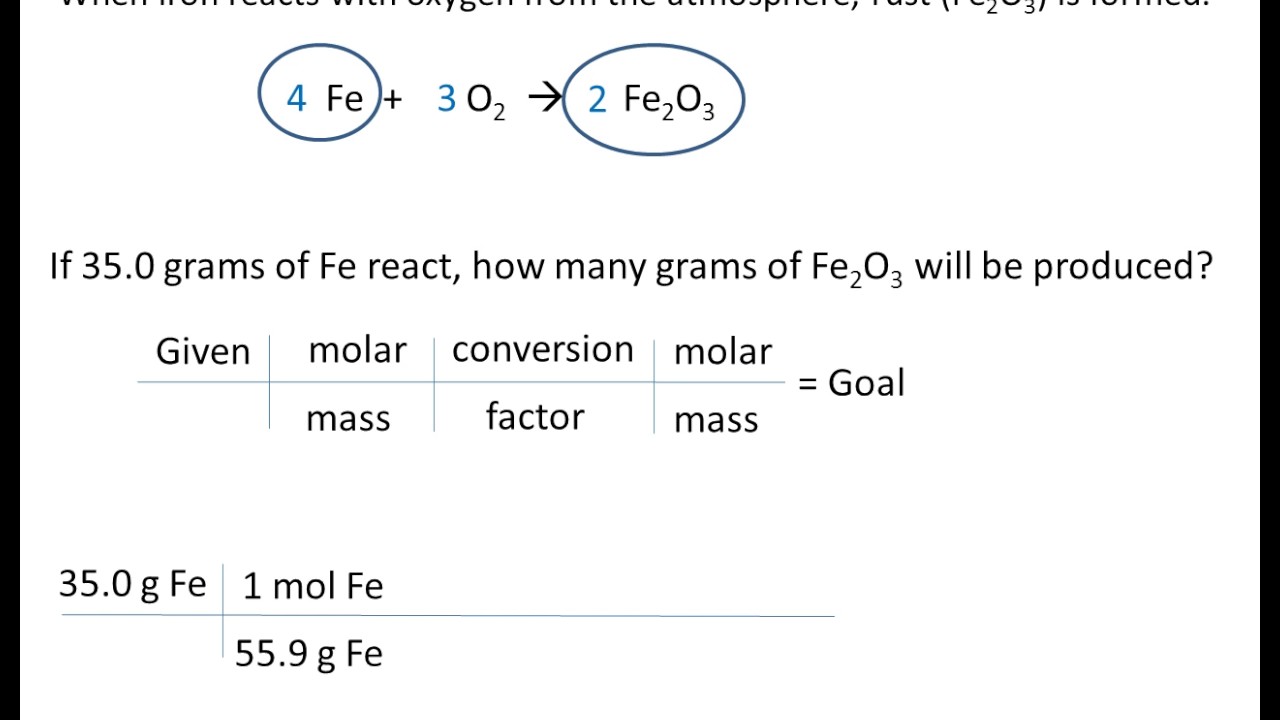
3 Stoichiometry Mass To Mass YouTube
https://i.ytimg.com/vi/X15NVr3KNRM/maxresdefault.jpg

Week 8 Stoichiometry Mass To Mass YouTube
https://i.ytimg.com/vi/n6GThx9lttI/maxresdefault.jpg
Mass To Mass Conversion Stoichiometry - Convert from mass of the species to moles of the species by dividing by the molar mass Use the stoichiometric ratio to convert from moles of one species to another Convert from moles back to mass by multiplying by the molar mass The molar mass of a species is the total mass of an element or compound per 1 mole