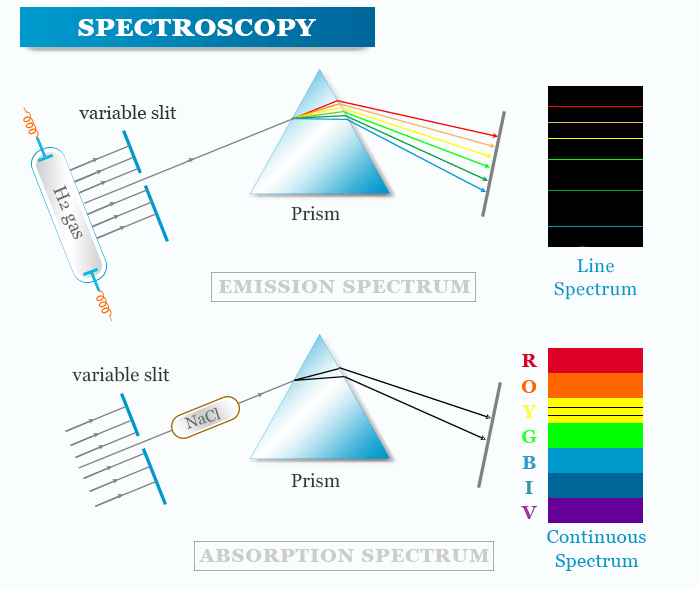
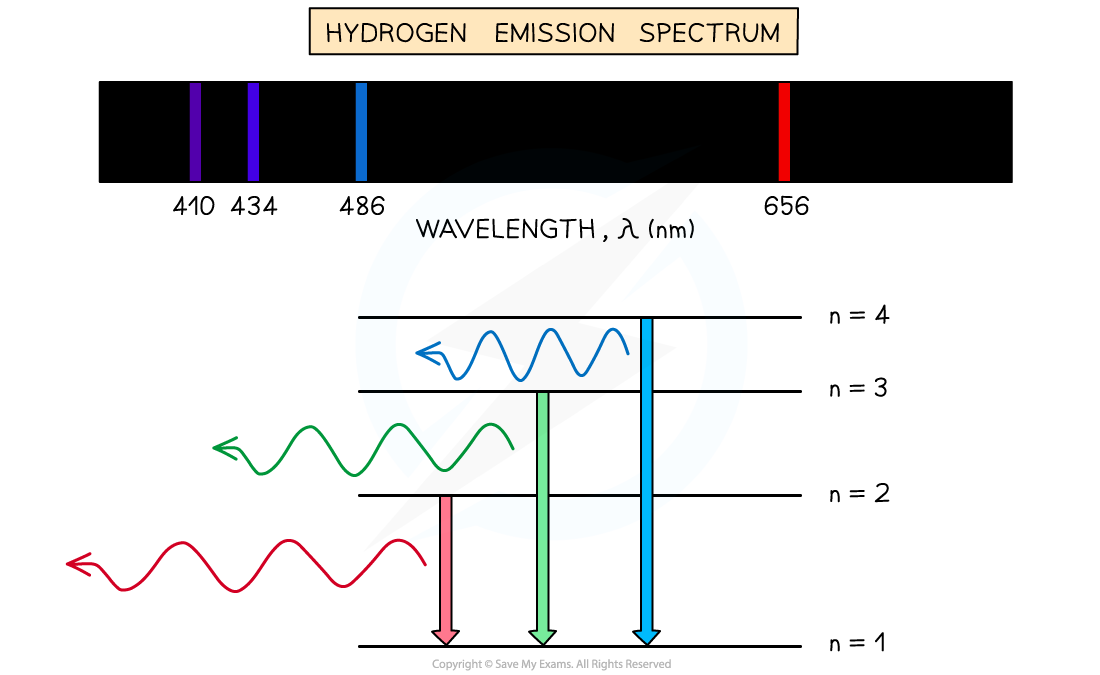
Line Emission Spectrum Simple Definition Chemistry When the emitted light is passed through a prism only a few narrow lines of particular wavelengths called a line spectrum are observed rather than a continuous range of wavelengths Figure 6 3 1 6 3 1
Jan 4 2025 nbsp 0183 32 A line spectrum also known as an emission spectrum is a type of spectrum that displays a series of lines or bands of light of different wavelengths and intensities In this The emission spectrum or line spectrum of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity When hydrogen gas is placed into a tube and electric current passed through
Line Emission Spectrum Simple Definition Chemistry

Line Emission Spectrum Simple Definition Chemistry
https://i.ytimg.com/vi/oFwTPMVYfpo/maxresdefault.jpg

Absorption And Emission Spectra IB And A Level Chemistry YouTube
https://i.ytimg.com/vi/lRx_-x2hpcM/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGH8gFCgTMA8=&rs=AOn4CLAbozb1czKgHEUkdG9LajrMoiIKIA

Lesson Explainer Emission And Absorption Spectra Nagwa 56 OFF
https://webbtelescope.org/files/live/sites/webb/files/home/resource-gallery/articles/_images/Spectroscopy/Article3/LabEmissionSpectra.jpg?t=tn2400
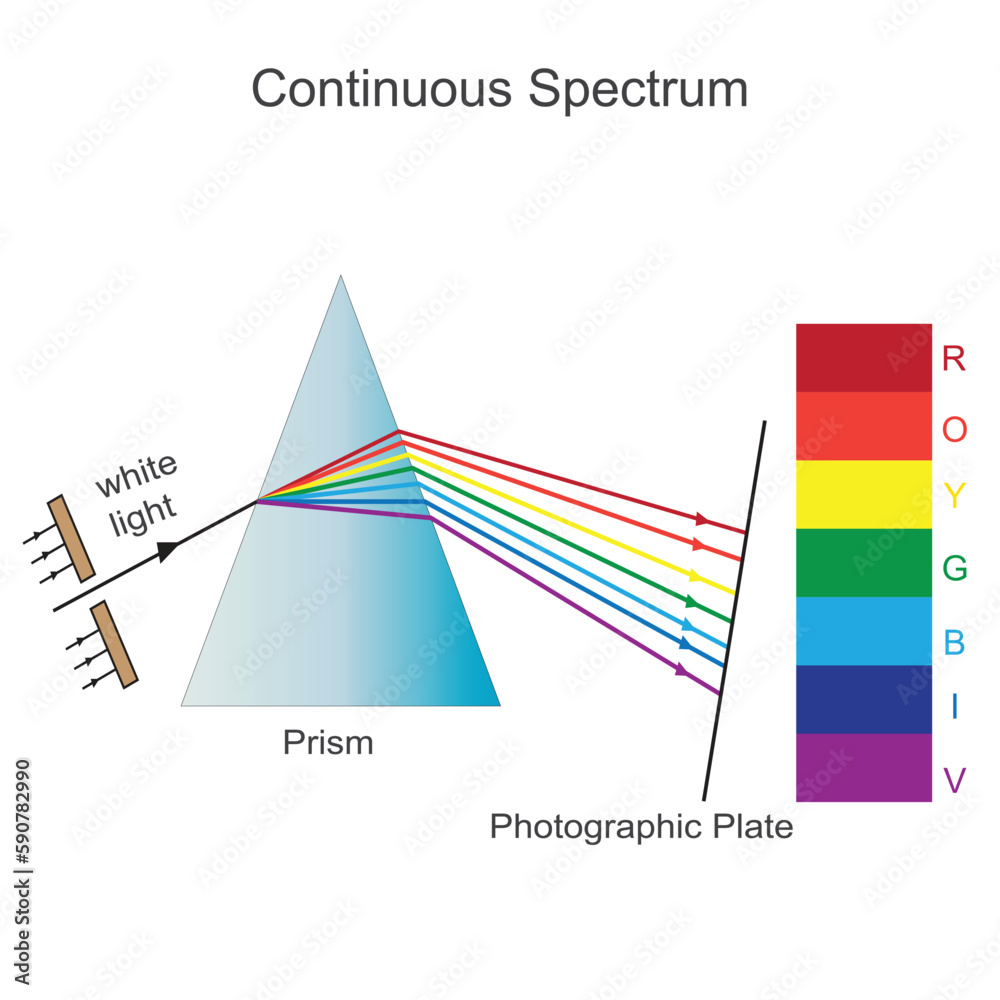
Sep 26 2022 nbsp 0183 32 The result is an emission spectrum that shows the intensity of emission as a function of wavelength The shapes of these emission spectra fall into two broad types line spectra and band spectra Nov 21 2023 nbsp 0183 32 What is a line emission spectrum Learn the characteristics of the line emission spectrum and how it is produced with examples
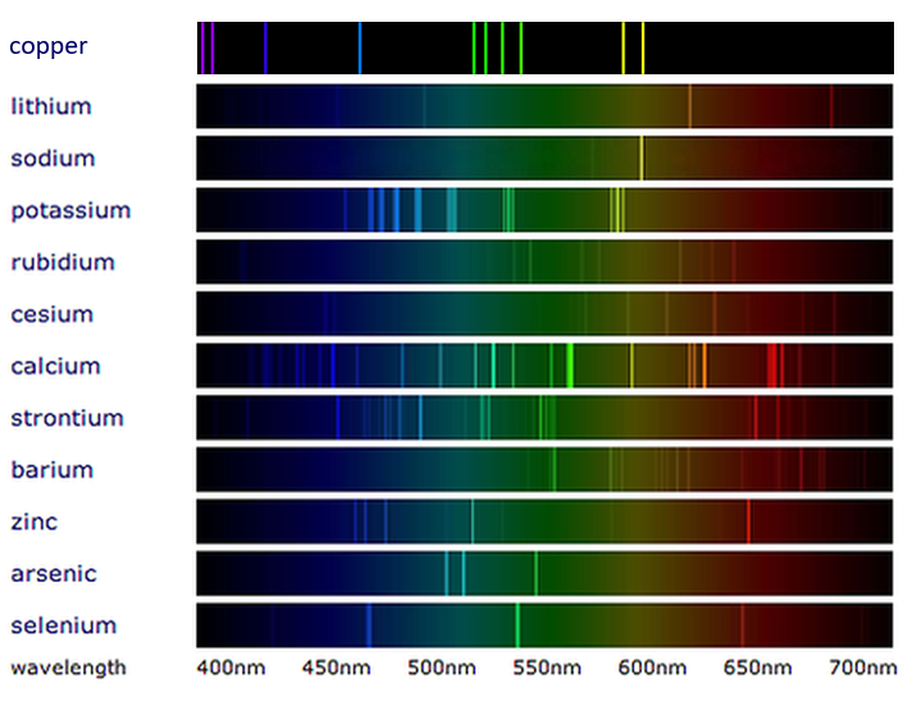
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to electrons making a transition from a Jul 3 2023 nbsp 0183 32 This spectrum is called line emission spectrum on line spectrum Each line in the spectrum corresponds to a particular wavelength Sodium always gives two yellow lines
More picture related to Line Emission Spectrum Simple Definition Chemistry

Define Emission And Absorption Spectrum Clearance Discounts Gbu
https://media.nagwa.com/709180464317/en/thumbnail_l.jpeg
Pasillitos De Hospital Diferencias Entre Asepsia Y 40 OFF
https://www.priyamstudycentre.com/wp-content/uploads/2022/11/Spectroscopy-definition-absorption-and-emission-types-of-electromagnetic-spectra-or-spectrum.png
Bright Line Spectra Mr Palermo s Flipped Chemistry Classroom
https://www.mrpalermo.com/uploads/9/8/9/6/9896107/published/screenshot-2021-01-05-091318.png?1609856043
So what is the emission spectrum definition in physics and chemistry An emission spectrum is the range or array of wavelengths spectra obtained when the light emitted by a substance is Line spectra result from electrons transitioning between energy levels in an atom Each element has a unique line spectrum serving as its fingerprint Emission spectra occur when electrons
May 20 2023 nbsp 0183 32 Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours The emitted light can be observed An emission spectrum is a series of lines formed when light emitted from an atom is focused by a slit and passes through a prism This process reveals discrete lines corresponding to different
Spectrum
https://as2.ftcdn.net/v2/jpg/05/90/78/29/1000_F_590782990_FI2lEELm5vbAX4L0iGOSiw5VwkJJFgrA.jpg
Edexcel A Level Physics 5 38 Atomic Line Spectra
https://oss.linstitute.net/wechatimg/2022/10/Hydrogen-Emission-Spectra.png
Line Emission Spectrum Simple Definition Chemistry - The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to electrons making a transition from a