Limiting Reagent And Percent Yield Worksheet 2 Answer Key WEB Answer Sheet 1 Consider the following reaction 3 NH4NO3 Na3PO4 NH4 3PO4 3 NaNO3 Answer the questions above assuming we started with 30 grams of ammonium
WEB When copper Il chloride reacts with sodium nitrate copper Il nitrate and sodium chloride are formed a Write the balanced equation for the reaction given above cuC12 NaN03 WEB Silver nitrate AgNO3 reacts with ferric chloride FeCl3 to give silver chloride AgCl and ferric nitrate Fe NO3 3 In a particular experiment it was planned to mix a solution
Limiting Reagent And Percent Yield Worksheet 2 Answer Key

Limiting Reagent And Percent Yield Worksheet 2 Answer Key
https://i.pinimg.com/originals/72/1e/11/721e11e4ca999356562b1224cb571672.jpg
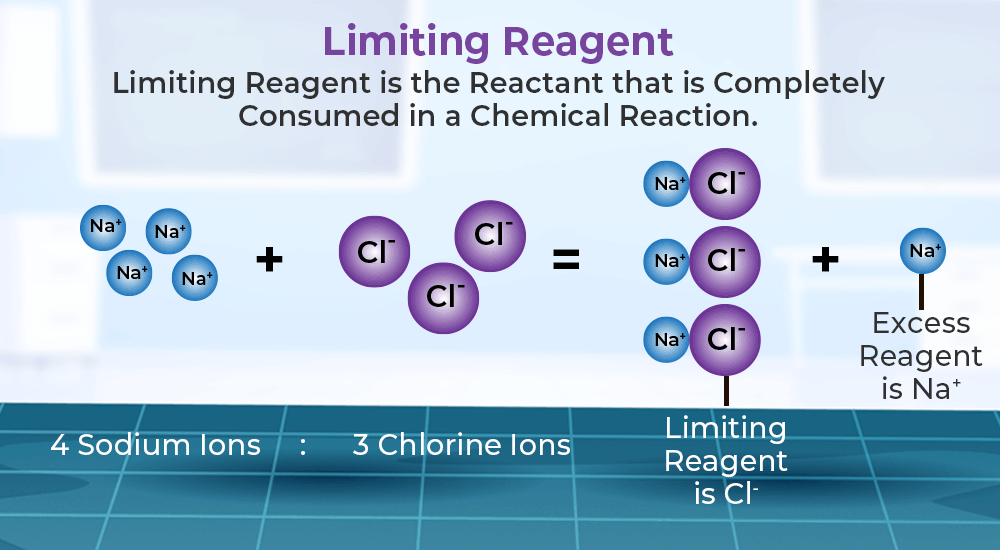
READ THE SCIENCE 12 3 Limiting Reagent And Percent Yield
https://media.geeksforgeeks.org/wp-content/uploads/20230321173440/Limiting-Reagent-2.png

Stoichiometry Limiting Reagent Worksheet Printable Word Searches
https://i2.wp.com/www.worksheeto.com/postpic/2015/10/percent-yield-worksheet-answers_224046.png
WEB This page titled Solutions Limiting Reagents Worksheet is shared under a CC BY NC SA 4 0 license and was authored remixed and or curated by Mark Draganjac via source content that was edited to the style and WEB 2 Write the equation for the reaction of iron III phosphate with sodium sulfate to make iron III sulfate and sodium phosphate a If you perform this reaction with 25 grams of iron III phosphate and an excess of sodium
WEB Determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation Use mole ratios to calculate the number of moles of product WEB Section 12 3 Limiting Reactants In your textbook read about why reactions stop and how to determine the limiting reactant Study the diagram showing a chemical reaction and the
More picture related to Limiting Reagent And Percent Yield Worksheet 2 Answer Key

Percent Yield Problems
https://s3.studylib.net/store/data/025183256_1-c3c2a032de1629d62f4cb160ef8765cd.png

Quiz Worksheet How To Calculate Percent Yield Study
https://study.com/academy/practice/quiz-worksheet-how-to-calculate-percent-yield.jpg
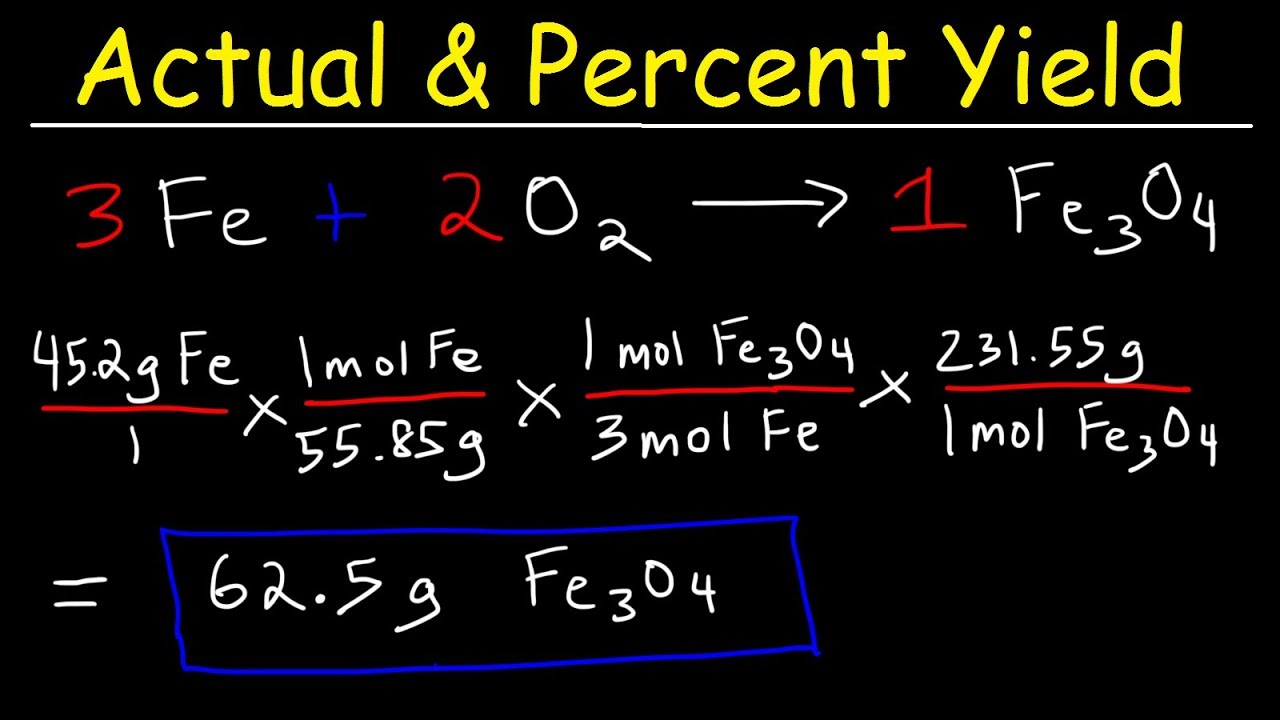
Percent Yield Actual Theoretical Yield Limiting Reagent
https://i.ytimg.com/vi/w1njGAkc8og/maxresdefault.jpg
WEB What is the theoretical yield in grams of aspirin C 9 H 8 O 4 when 2 g of C 7 H 6 O 3 is heated with 4 g of C 4 H 6 O 3 If the actual yield of WEB The overall document is a worksheet to practice skills in limiting reactants theoretical yields and percent yields for multiple chemical reactions This document provides 5 chemistry problems involving calculating
WEB Apr 6 2010 nbsp 0183 32 This document discusses limiting reactants theoretical yield actual yield and percent yield in chemical reactions It defines limiting reactant as the reactant that limits the amounts of other reactants that WEB The document provides examples of stoichiometry calculations involving limiting reagents theoretical and percentage yields It includes calculations for several chemical reactions

Percent Yield Worksheet Answers Chemistry A Study Of Matter
https://i0.wp.com/www.chemistryworksheet.com/wp-content/uploads/2022/10/percent-yield-worksheet-2.png?resize=768%2C994&ssl=1

How To Calculate Percent Yield In Chemistry 15 Steps Chemistry
https://i.pinimg.com/originals/e9/4d/28/e94d2894506f2d96de90b204e87e5a0e.jpg
Limiting Reagent And Percent Yield Worksheet 2 Answer Key - WEB Limiting Reactant And Percent Yield Worksheet Answers Recipes LIMITING REACTANT AND PERCENT YIELD PRACTICE CCHS Answer Sheet Consider the following