Limiting Reactant And Percent Yield Practice Answer Key The limiting reactant is the reactant from which the minimum amount of product is formed Also if we calculate the amount of one reactant needed to react with another reactant then the
Criticism of Law of Limiting Factors While explaining the principle of limiting factors Blackman exhibited abrupt breaks in the rate of photosynthesis due to the low intensity of light According What is Kohlrausch law of independent migration According to Kohlrausch s law of independent ion movement the limiting molar conductivity of an electrolyte can be described as the sum
Limiting Reactant And Percent Yield Practice Answer Key

Limiting Reactant And Percent Yield Practice Answer Key
https://s3.studylib.net/store/data/025233975_1-ecd8207330556e3925e53e2ccde87078-768x994.png

Limiting Reagent Questions And Answers Pdf
https://i.pinimg.com/originals/e1/20/fb/e120fb80329e0f9a11bc5565bd6c6679.jpg

141 Limiting Reactant Worksheet Key Limiting Reactant Theoretical
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/586887c251a2912d538ac38d813f94b7/thumb_1200_1553.png
What is a limiting reactant The reactant that is fully consumed or entirely reacted in a reaction is known as a Limiting Reactant What is the difference between theoretical and actual yield Theoretical yields are calculated amounts of products based on the complete reaction of the limiting reagent whereas the actual
In Mathematics a limit is defined as a value that a function approaches the output for the given input values Limits are important in calculus and mathematical analysis and used to define A limiting reagent is a reactant that occursin lower concentrations in a reaction When it is consumed the reaction will stop regardless of the amount of reactant present in the reaction
More picture related to Limiting Reactant And Percent Yield Practice Answer Key

42 Limiting Reactant Worksheet Answers Worksheet Resource
https://db-excel.com/wp-content/uploads/2019/09/limiting-reagent-worksheet-5.png
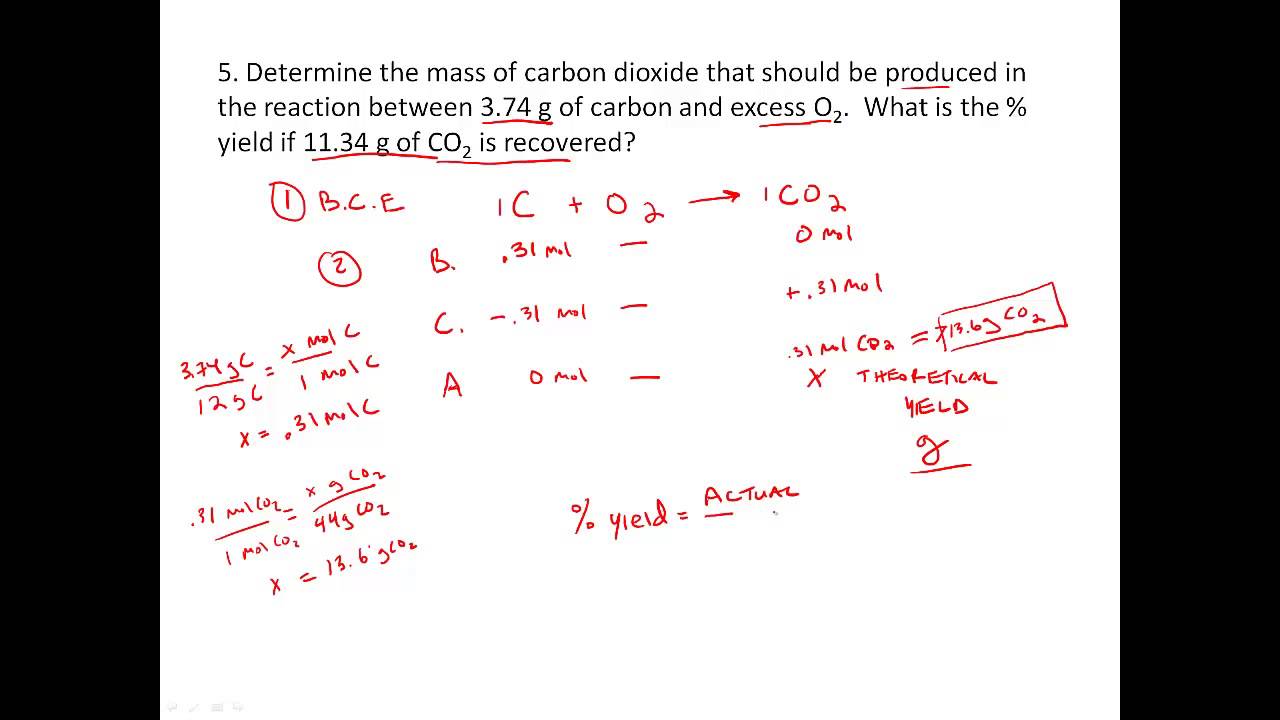
Stoichiometry Practice Percent Yield Problems YouTube
https://i.ytimg.com/vi/aB_S-C02bgM/maxresdefault.jpg

Limiting Reagent Worksheet 1 Answers In 2022 Persuasive Writing
https://i.pinimg.com/originals/52/dd/4d/52dd4d3cd34dbca71302417c2846ac71.png
The limiting friction hinges upon the material the nature of the surfaces interacting and their evenness So long as the normal reaction is the same the magnitude of limiting friction is free The value of limiting molar conductivity 203 176 m can be determined from the graph or with the help of Kohlrausch law The general equation for the plot is given as For strong electrolytes the molar
[desc-10] [desc-11]

Limiting Reactants Worksheet
https://s3.studylib.net/store/data/008633156_1-38ac515997cae06d32815e09db3664f0.png

Limiting Reactant And Percent Yield Practice Worksheet Answer Key
https://i.ytimg.com/vi/s434qOiWyNA/maxresdefault.jpg
Limiting Reactant And Percent Yield Practice Answer Key - A limiting reagent is a reactant that occursin lower concentrations in a reaction When it is consumed the reaction will stop regardless of the amount of reactant present in the reaction