Limitations Of Balanced Chemical Equation Class 10 It does not inform about the time taken for the completion of the reaction It does not inform about the rate at which a reaction proceeds It does not inform about the heat changes during the reaction i e whether the heat is given out or absorbed
The limitations of a balanced chemical equation are as follows 1 The balanced chemical equation does not give the actual yield of the product 2 The balanced chemical equation does not give the information about the rate of the chemical reaction 3 The balanced chemical equation does not tell the physical state of the reactants and the In conclusion while chemical equations are a useful tool for representing chemical reactions they have several limitations that must be taken into account It is important to understand these limitations in order to interpret and apply chemical equations correctly
Limitations Of Balanced Chemical Equation Class 10

Limitations Of Balanced Chemical Equation Class 10
https://i.ytimg.com/vi/bXIR7go_b38/maxresdefault.jpg

Balanced Chemical Equation Chemical Reaction And Equation Science
https://i.ytimg.com/vi/t86MHDAAgNI/maxresdefault.jpg

Class 10 Chemical Reactions And Equations Important Questions And
https://www.cbseguidanceweb.com/wp-content/uploads/2022/12/chemical-reactions-and-equations-class-10-important-questions-and-answers-2023-24-1-1024x576.png
Limitations of chemical equations The chemical reactions suffer from a number of limitations and thus have a few drawbacks also The physical states of the reactants and products Feb 26 2019 nbsp 0183 32 Following are the limitations of a chemical equation i A chemical equation does not tell us the physical state of the reactants and the products in the reaction ii It does not tell us the actual concentration or dilution of the reactants used in the reaction
Aug 10 2021 nbsp 0183 32 What is a balanced chemical equation Why should chemical equations be balanced Solution An equation for a chemical reaction is an equation in which the number of atoms for each element in the reaction and the total charge is Dec 16 2024 nbsp 0183 32 An unbalanced chemical equation is not an accurate representation of a chemical equation and thus it requires balancing Chemical reactions should be balanced in order to obey the law of conservation of mass which states that mass can neither be created nor be destroyed
More picture related to Limitations Of Balanced Chemical Equation Class 10

Chemical Reactions And Equation Balanced Equation Class 10
https://i.ytimg.com/vi/X0Y356WXrNo/maxresdefault.jpg

Chemical Reactions And Equations Session 1 Balancing Of Chemical
https://i.ytimg.com/vi/uRnZxmCOxsI/maxresdefault.jpg

What Is A Balanced Chemical Equation Why Should Chemical Equations Be
https://i.ytimg.com/vi/e61XzaW6GBo/maxresdefault.jpg
Dec 16 2024 nbsp 0183 32 Explanation In a balanced chemical equation the number of atoms of each element is balanced on the left and right side of the equation Na s H 2 O l NaOH aq H 2 g Unbalanced equation Write the chemical formulae of X and Y Write a balanced chemical equations when X and Y are dissolved in water Complete and balance the following equation ce C2H5COONa NaOH gt Delta CaO
Any 4 limitations of balanced chemical eqn are The physical estate of reactants and products The concentration of reactants The rate of the chemical reaction The time is taken to complete the reaction State the main limitations of a chemical equation Physical states of the reactants or products But now we write along with substances l for liquids s for solid and g for gas Conditions that affect the reaction i e temp pressure or catalyst Concentration of reactants and products we use dil for dilute and cone for concentrated
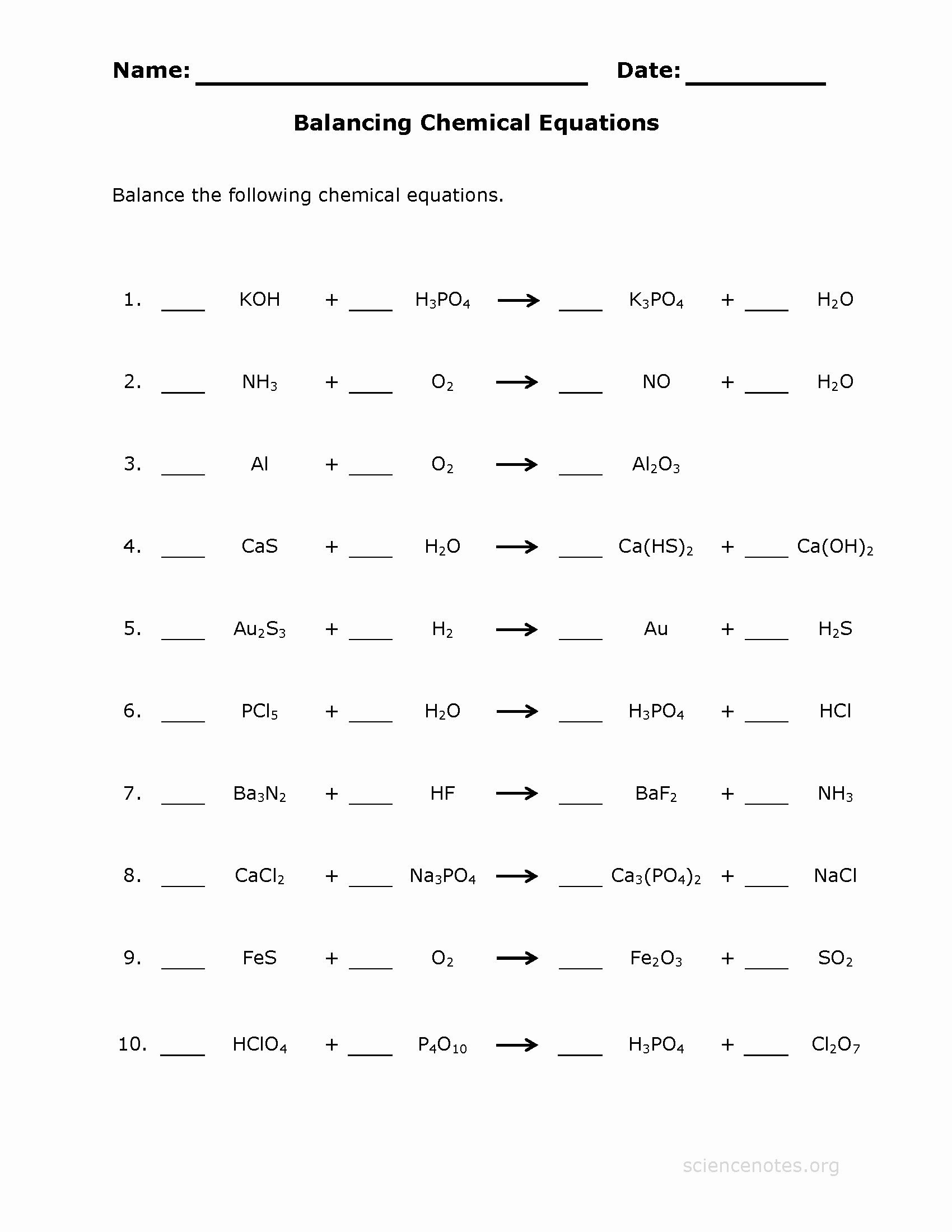
Practice Balancing Equations Worksheets
https://chessmuseum.org/wp-content/uploads/2019/10/balancing-equation-worksheet-with-answers-fresh-balancing-chemical-equations-practice-sheet-of-balancing-equation-worksheet-with-answers.png

P8 Chemistry Blog Brad Accelerated Chemistry Blog 4
http://www.wikihow.com/images/2/2f/Balance-Chemical-Equations-Step-6-preview.jpg
Limitations Of Balanced Chemical Equation Class 10 - Aug 10 2021 nbsp 0183 32 What is a balanced chemical equation Why should chemical equations be balanced Solution An equation for a chemical reaction is an equation in which the number of atoms for each element in the reaction and the total charge is