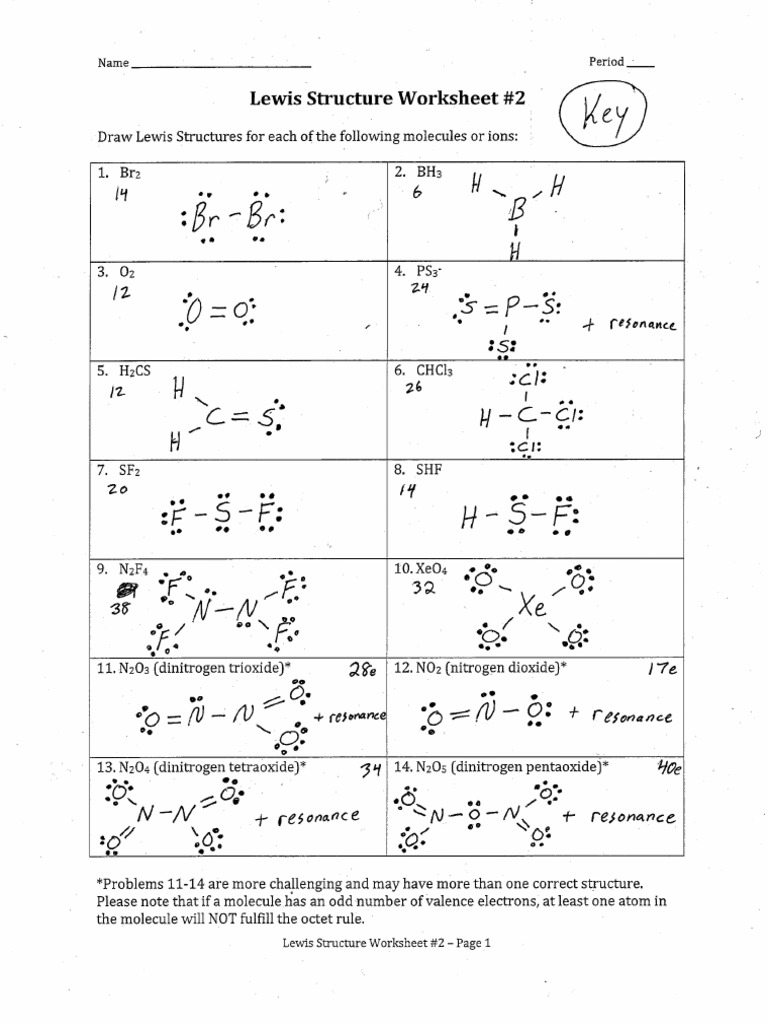
Lewis Dot Structures Worksheet Answers Pdf Lewis Structure Worksheet Answer Key Dr Scott Beaver Name Date Page 1 of 3 1 Draw a Lewis dot structure for each molecule CF 4 CH 4 CH 3 F CH 2 F 2 C F F F F C H H H H C H H H F C H F H F F in any position F in any position Cl Cl Lewis structure Worksheet Answer Key H 2 O NH 3 PCl 3 H 2 S H 2 F 2 H O H N H H H Dr Scott Beaver Page 2 of 3
Draw three possible Lewis structures for N 2O and assign formal charges to the atoms in each molecule Then identify the most stable structure for N 2 O Note N must be the Practice Sheet Electron Dot Lewis Structures A Lewis or Electron Dot Structure is a convenient representation of the valence electrons in an atom An electron dot structure for an atom is simply the symbol for the element surrounded by a number of dots equal to the number of valence electrons
Lewis Dot Structures Worksheet Answers Pdf
Lewis Dot Structures Worksheet Answers Pdf
https://s2.studylib.net/store/data/010212602_1-7d40b2312f1ea3c3e061ca00f159c411.png
Chemistry Worksheet Lewis Dot Structures Answers Promotiontablecovers
https://i0.wp.com/imgv2-1-f.scribdassets.com/img/document/360479464/original/76f49666cd/1516979920?v=1

Lewis Structure Worksheet With Answers
https://s3.studylib.net/store/data/008803694_1-89b0d8b65c5fb956d422554195147920.png
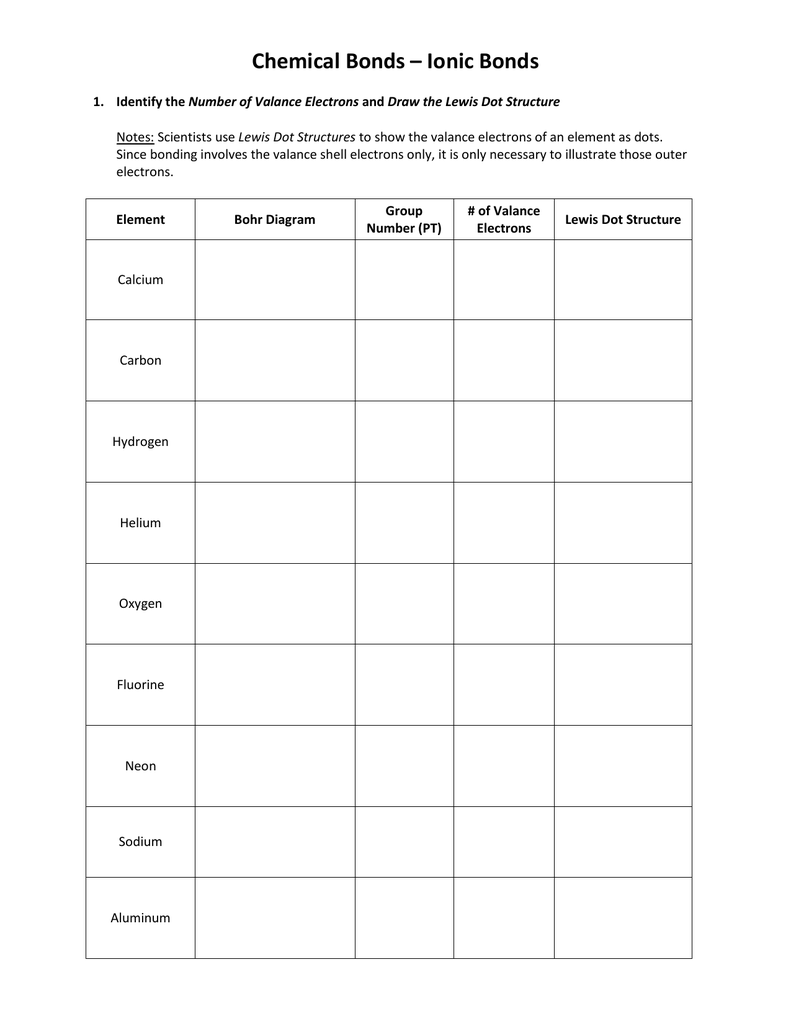
Answer Key How to Draw a Lewis Dot Structure Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion You can quickly refer to the periodic table for the group A number for this information Lewis Dot Structure Mega Worksheet 1 What is the octet rule Explain its role in bonding between atoms 2 Indicate how many electrons must be gained or lost by each of the following atoms to achieve a stable electron configuration e g 3 lost 2 gained etc a Sr b Sb c Si d S e Se f Xe llowing pairs of elem
Lewis Dot Structure Worksheet In the first box below the formula write your first impression of the Lewis formula In the second box calculate S 2 B and identify bonding around central atom CHEM 1A VSEPR Theory Now that we have an understanding of covalent bonding and how atoms share electrons to form molecules and polyatomic ions we will use Lewis dot structures to predict electronic and molecular geometries
More picture related to Lewis Dot Structures Worksheet Answers Pdf

Lewis Dot Structures Drawing Resonance Examples Video Lesson
https://study.com/cimages/videopreview/thumb_133311.jpg
50 Lewis Dot Structure Practice Worksheet
https://chessmuseum.org/wp-content/uploads/2019/10/lewis-dot-structure-practice-worksheet-luxury-lewis-structures-worksheet-video-worksheet-with-answers-of-lewis-dot-structure-practice-worksheet.jpg

3 Ways To Draw Lewis Dot Structures WikiHow
http://www.wikihow.com/images/a/a1/Draw-Lewis-Dot-Structures-Step-13.jpg
Chemistry Worksheet Lewis Dot Structures Name Block 2 Draw Lewis structures for the following covalent compounds Created Date 3 28 2014 11 29 01 AM Lewis Structure Worksheet Dr Scott Beaver Name Date Page 1 of 3 1 Draw a Lewis dot structure for each molecule CF 4 CH 4 CH 3 F CH 2 F 2
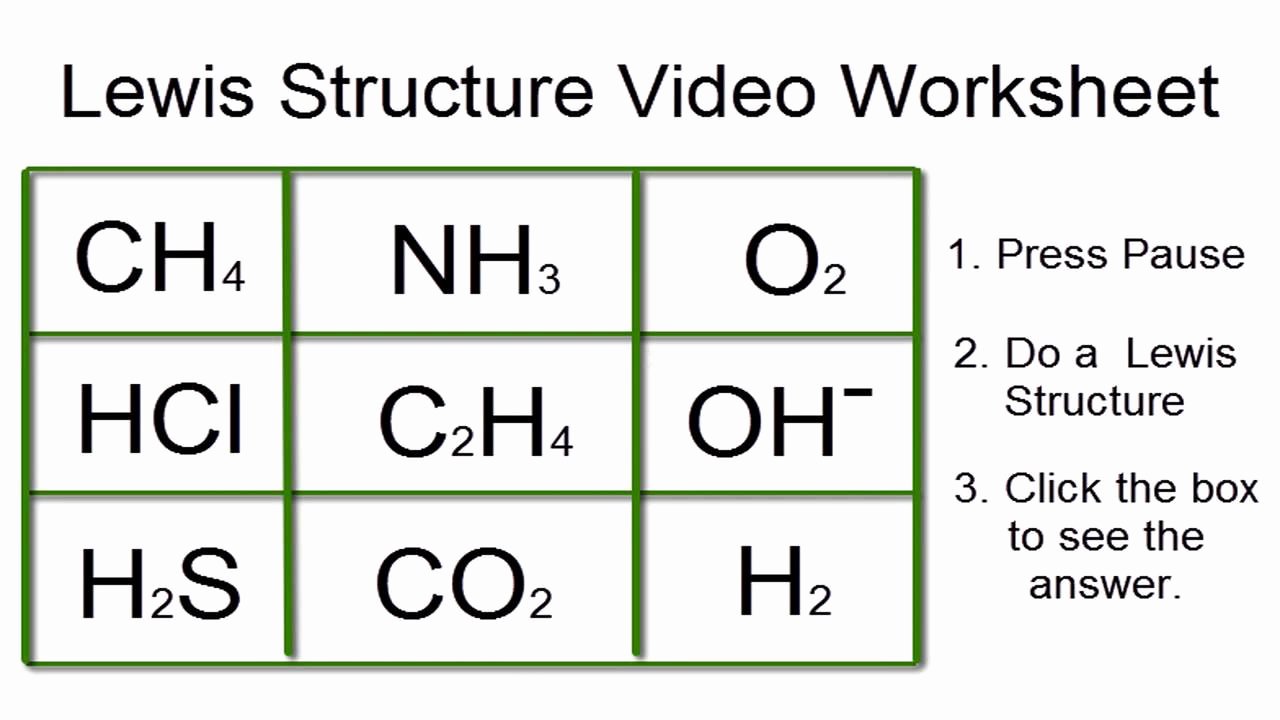
Draw the Lewis dot structure for each of the following polyatomic ions a NH4 c PO4 3 b NO3 d CO3 2 4 For the following molecules or ions where the central atom is underlined Draw the Electron dot structure Determine the shape of the molecule Determine the approximate bond angles CH2F2 OF2 phosphite ion PO3 3 5 Covalent Bonds and Molecular Structure 1 How are ionic bonds and covalent bonds different 2 Describe the relationship between the length of a bond and the strength of that bond
ChemE Lewis Structure Worksheet 2 Answers PDF
https://imgv2-1-f.scribdassets.com/img/document/440137550/original/5af75ba11c/1628609361?v=1

Lewis Electron Dot Diagram Worksheet
https://s2.studylib.net/store/data/010001948_1-d42cec3a405cb0abadea501d80316357-768x994.png
Lewis Dot Structures Worksheet Answers Pdf - Answer Key How to Draw a Lewis Dot Structure Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion You can quickly refer to the periodic table for the group A number for this information