Le Chatelier S Principle Worksheet Answers Pdf Le Ch 226 telier s Principle Worksheet 1 What would happen to the position of the equilibrium when the following changes are made to the equilibrium system below CH 4 g 2H 2 S g CS 2 g 4H 2 g a Decrease the concentration of dihydrogen sulfide hydrosulfuric acid Equilibrium will shift to favor reactants
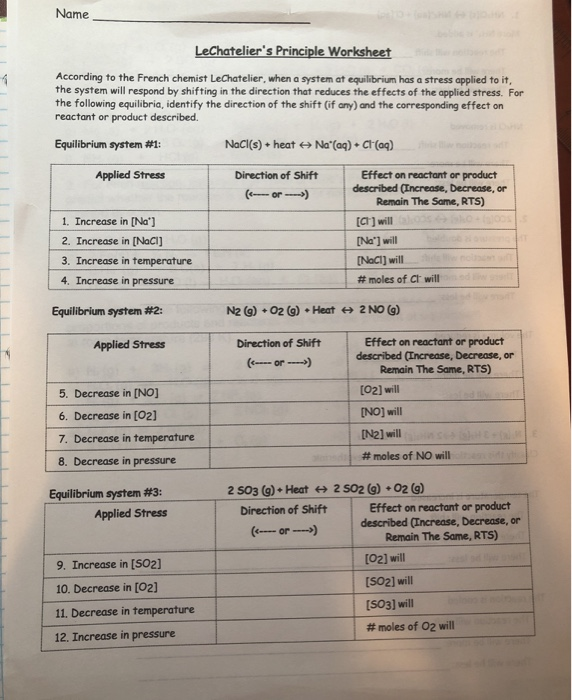
Le Chatelier s Principle states that when a system at equilibrium is subjected to a stress the system will shift its equilibrium point in order to relieve the stress Complete the following chart by riting left right or none r equilibrium shift and Le Chatelier s Principle allows us to predict the results that follow from changing the conditions of a system at chemical equilibrium This allows scientists to develop techniques to control chemical reactions in natural and industrial settings in order to obtain desired products
Le Chatelier S Principle Worksheet Answers Pdf
Le Chatelier S Principle Worksheet Answers Pdf
https://media.cheggcdn.com/study/0b1/0b135d26-f047-4944-b05e-c15a2633a247/image.png
Le Chateliers Principle Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/05/Le-Chateliers-Principle-Changing-Temperature-at-Equilibrium.png

Le Chatelier s Principle
https://sciencenotes.org/wp-content/uploads/2022/10/Le-Chateliers-Principle-768x512.png
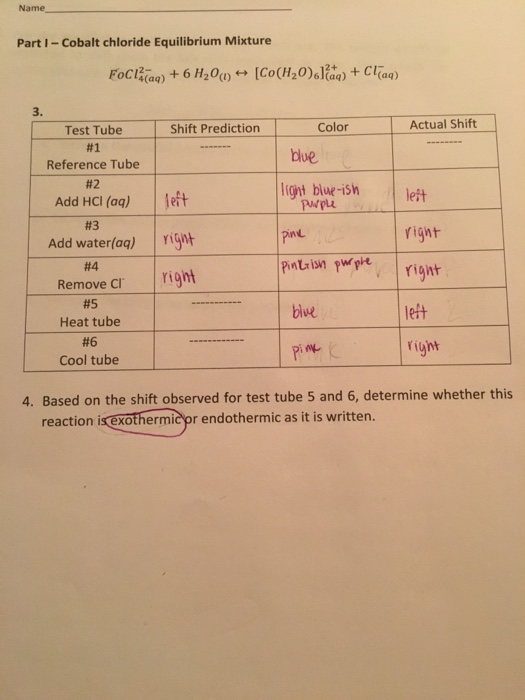
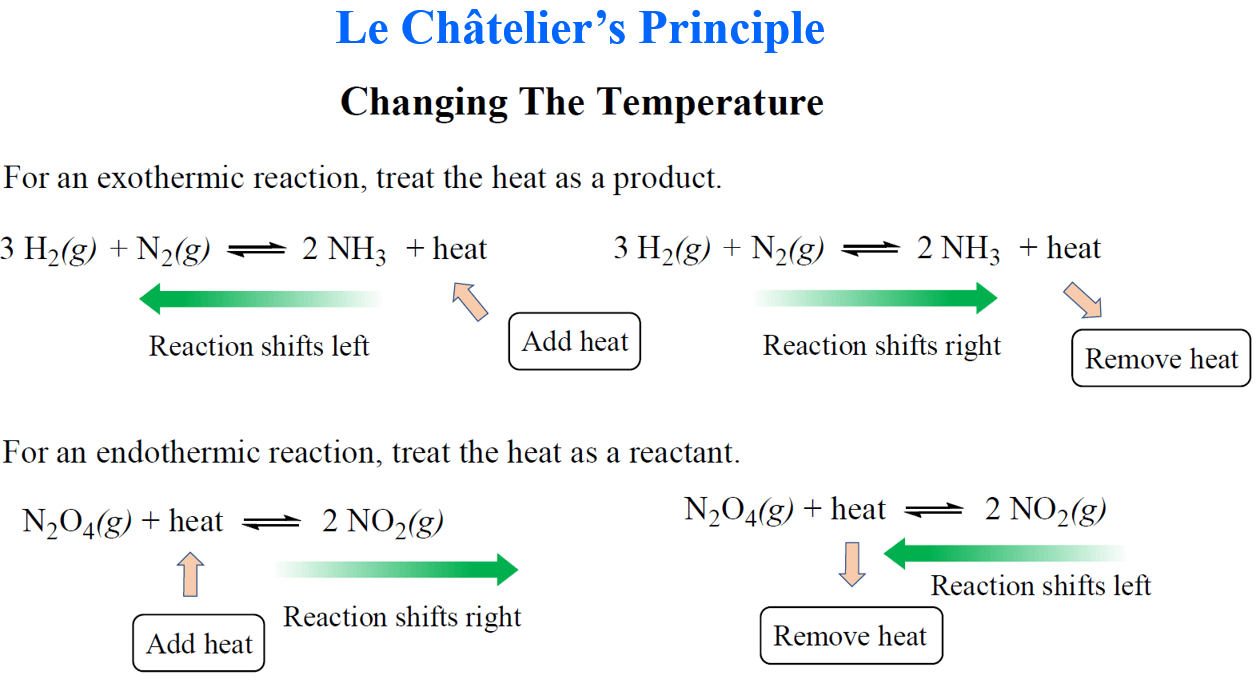
Worksheet 2 LeChatelier s Principle Describe the changes that occur after each stress is applied to the equilibrium N 2 g 3H 2 g 2NH 3 g 92 KJ Shifts Shifts to the Stress N 2 H 2 NH 3 Right or Left Reac Prod 1 N 2 is Worksheet Le Chatelier s Principle Name CO 9 1 120 g 3 In this reaction CO g H g heat a Is heat absorbed or released by the forward reaction b In which direction will the equilibrium shift if these changes are made
1 State Le Ch 226 telier s Principle 2 marks When a stress is imposed to a system at equilibrium the system will shift to oppose the stress and re establish equilibrium 2 For the reaction PCl3 g Cl2 g PCl5 g H 92 5 kJ predict the effect on the position of equilibrium that results from 6 marks Stress Shift Stress Shift Equilibrium Le Chatelier s Principle and Keq Practice 23 For the equilibrium system described by 2SO 2 g O 2 g 2SO 3 g at a particular temperature the equilibrium concentrations of SO 2 O 2 and SO 3 were 0 75 M 0 30 M and 0 15 M respectively At the temperature of the equilibrium mixture calculate the equilibrium constant
More picture related to Le Chatelier S Principle Worksheet Answers Pdf

Le Chatelier Worksheet Answers 17 19 YouTube
https://i.ytimg.com/vi/axTsQ7slRco/maxresdefault.jpg

Reversible Reactions Equilibrium And Le Ch telier s Principle
https://scitechconnect.elsevier.com/wp-content/uploads/2017/05/Reversible-Reactions-Equilibrium-and-Le-Chateliers-Principle.jpg

OneClass Any Help explanations With This Le Chatelier s Principle Lab
https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/130/13018266.png
Le Chatelier s Principle Worksheet 1 For the reaction below which change would cause the equilibrium to shift to the right CH4 g 2H2S g CS2 g 4H2 g a Decrease the concentration of dihydrogen sulfide b Increase the pressure on the system c Increase the temperature of the system Le Chatelier s Principle Worksheet 1 For the reaction below which change would cause the equilibrium to shift to the right in this endothermic reaction CH 4 g 2H 2 S g CS 2 g 4H 2 g a Decrease the concentration of dihydrogen sulfide b Increase the pressure on the system c Increase the temperature of the system
[desc-10] [desc-11]
Use Le Chatelier s Principle To Explain The Different Colors Desmond
https://media.cheggcdn.com/media/925/925ff0d7-9876-41e1-8f68-7f05f59f4da6/image

Le Chatelier s Principle Chemistry Practice Worksheet CHEM 1060 U
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/451b8f47215bad36373a6ab502164f13/thumb_1200_1553.png
Le Chatelier S Principle Worksheet Answers Pdf - Equilibrium Le Chatelier s Principle and Keq Practice 23 For the equilibrium system described by 2SO 2 g O 2 g 2SO 3 g at a particular temperature the equilibrium concentrations of SO 2 O 2 and SO 3 were 0 75 M 0 30 M and 0 15 M respectively At the temperature of the equilibrium mixture calculate the equilibrium constant