Law Of Conservation Of Mass Nov 21 2023 nbsp 0183 32 The Law of the Conservation of Mass was first realized by Antoine Lavoisier in the late 18th century It states that the total mass in the system is the same before and after the reaction
Print The Law of Conservation of Mass Definition Formula amp Examples Worksheet 1 In a chemical reaction 300 grams of reactant A are combined with 100 grams of reactant B Both A and B react Make predictions about the starting mass of the reactants vs the ending mass of the products in a chemical reaction based on the Law of Conservation Explain how Lavoisier came to discover the
Law Of Conservation Of Mass

Law Of Conservation Of Mass
https://d1avenlh0i1xmr.cloudfront.net/259f6a1a-f94d-4a3a-880c-0107b9944766/law-of-conservation-of-mass.-2-image-teachoo.jpg
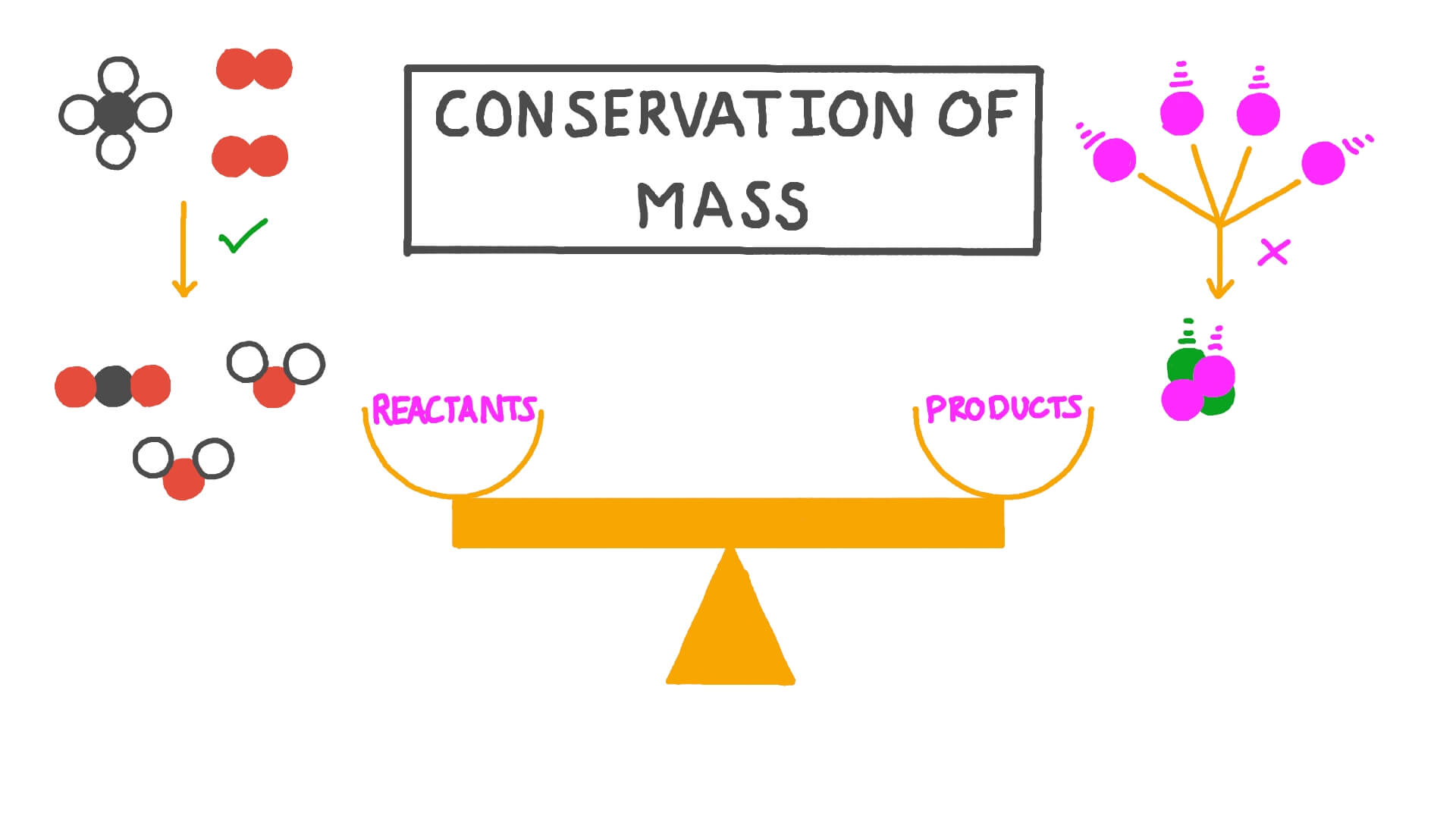
Law Of Conservation Of Mass The Law Of Conservation Of Mass Stock
https://media.nagwa.com/418127413712/en/thumbnail_l.jpeg
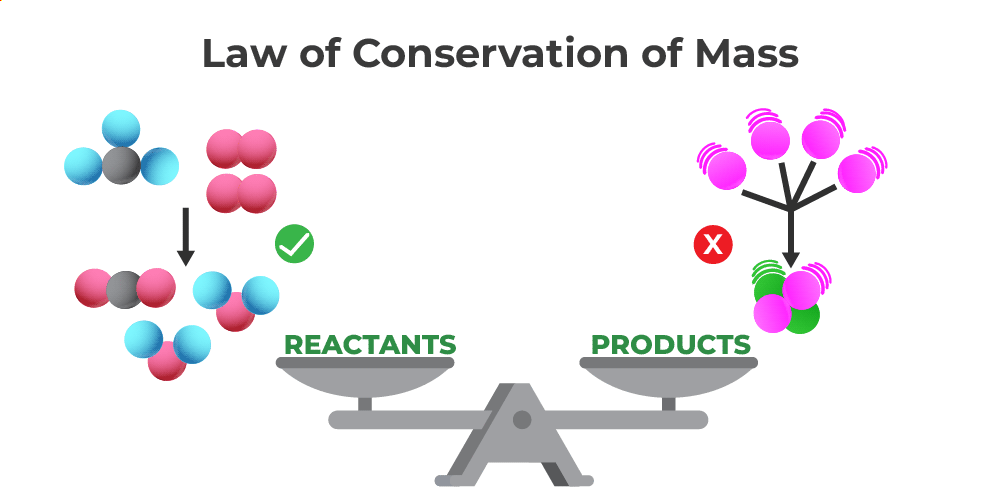
Law Of Conservation Of Mass Definition Formula Solved Examples
https://media.geeksforgeeks.org/wp-content/uploads/20221109111142/LawofConservationofMass.png
Nov 21 2023 nbsp 0183 32 The First Law of Thermodynamics is an extension of the Law of Conservation of Energy for thermodynamic systems Consider that eq Delta Q eq amount of heat is supplied to a system Answer and Explanation 1 The law of conservation of mass states that mass has to be accounted for in any type of reaction No mass can be lost and no mass can be gained
The law of conservation of mass will not be strictly valid unless mass and energy are considered together Law of conservation of mass implies that mass can neither be created nor destroyed although it may be rearranged in space or the entities associated with it may be changed in form For example in chemical reactions the mass of the chemical components before the reaction is equal to the mass of the components after the reaction
More picture related to Law Of Conservation Of Mass
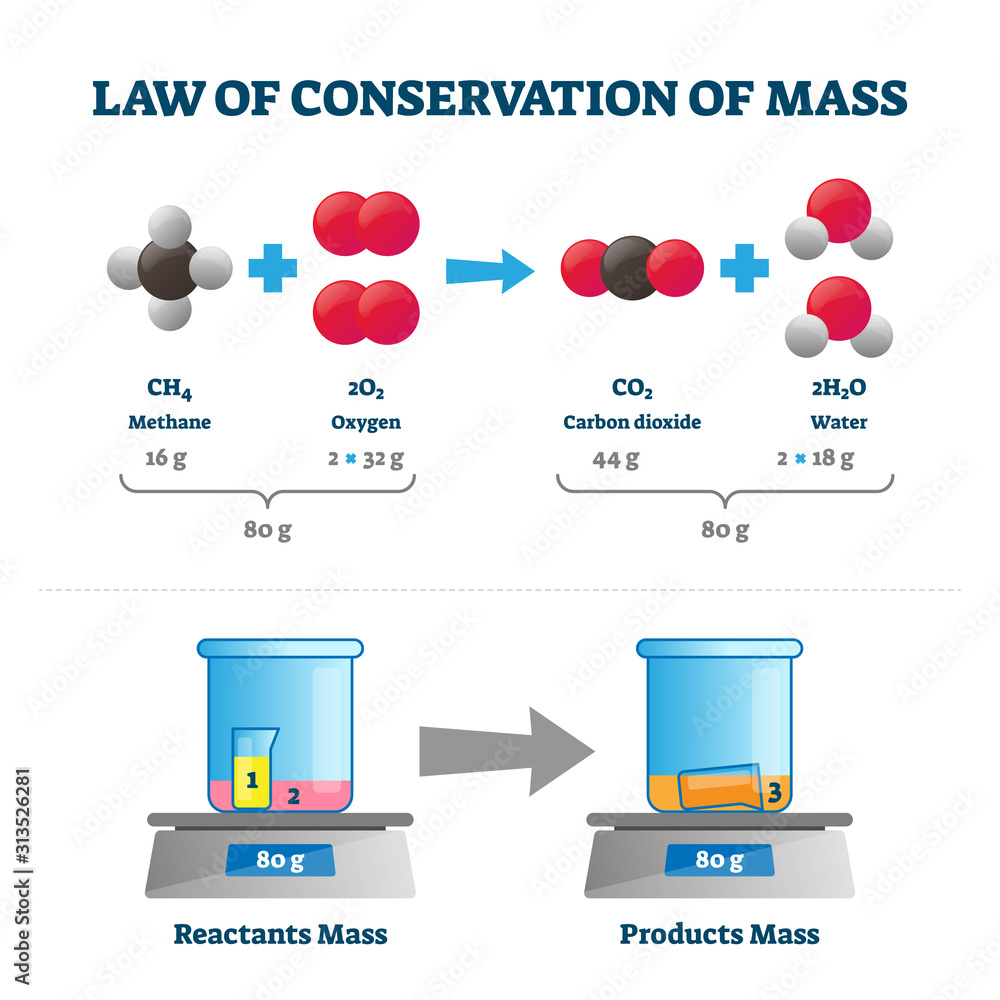
Law Of Conservation Of Mass Vector Illustration Labeled Educational
https://as1.ftcdn.net/v2/jpg/03/13/52/62/1000_F_313526281_4xxnARBcfQtLeLHkIFjcT8CDXUHnkjJf.jpg

The Law Of Conservation Of Mass Definition Equation Examples My XXX
https://www.flexiprep.com/NCERT-Exemplar-Solutions/Science/Class-9/posts/Ch-3-Atoms-And-Molecules-Part-11/Image-of-Law-of-Conservation-of-mass.png
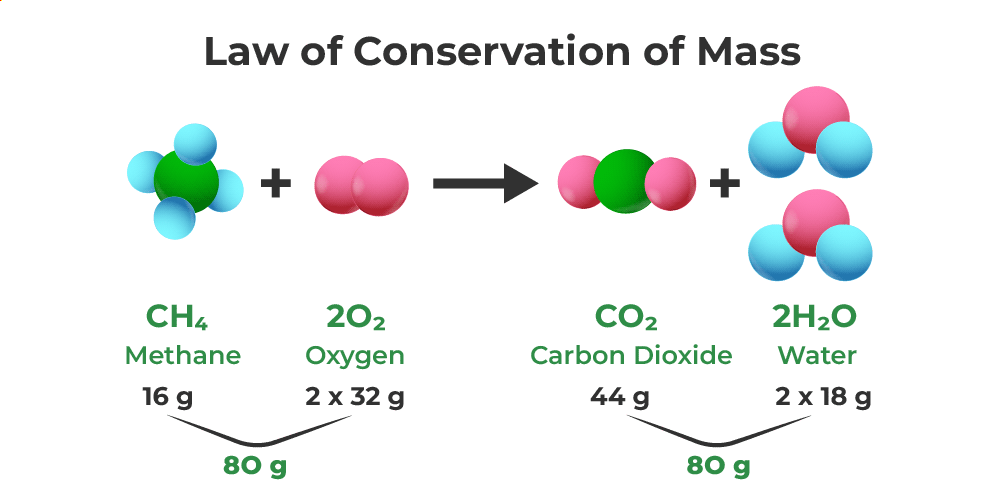
Law Of Conservation Of Energy Equation Chemistry
https://media.geeksforgeeks.org/wp-content/uploads/20221109111602/LawofConservationofMassExample2.png
If of conservation of mass was to hold true then 20 8 g of B a C l 2 on reaction with 9 8 g of H 2 S O 4 will produce 7 3 g of H C l and B a S O 4 equal to View Solution Q 3 Conservation of Mass Lesson Plan Joanne has taught middle school and high school science for more than ten years and has a master s degree in education The law of conservation of mass is an
[desc-10] [desc-11]
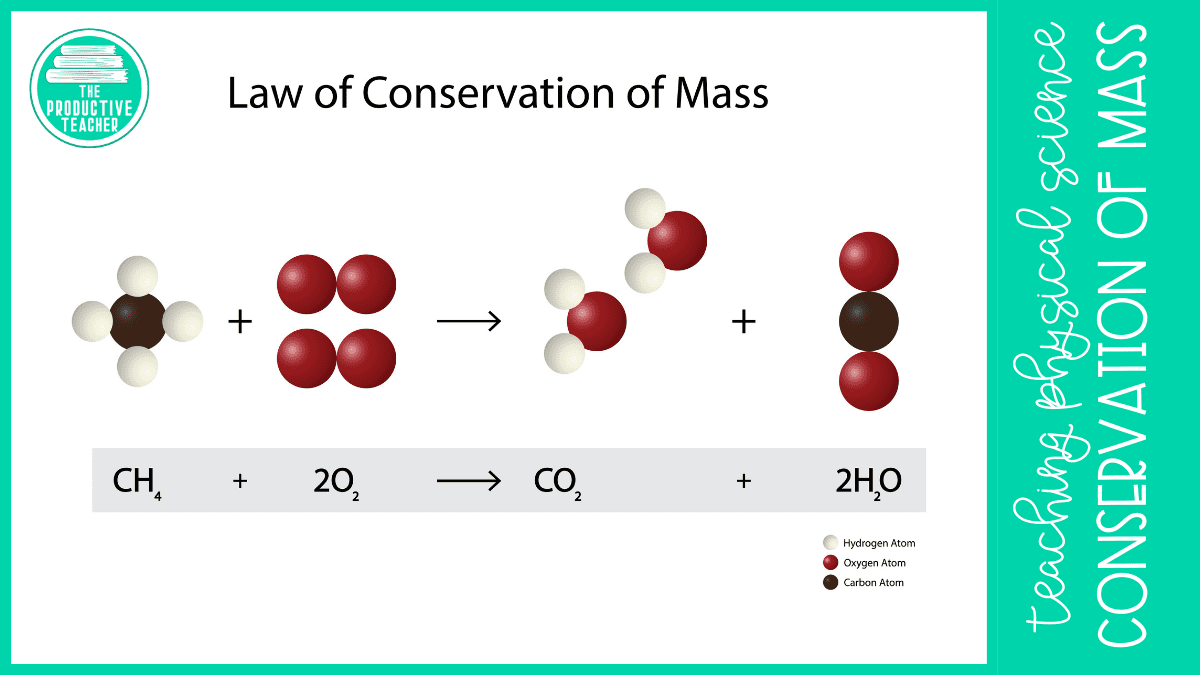
Law Of Conservation Of Mass Picture What Is The Law Of Conservation Of
https://theproductiveteacher.com/wp-content/uploads/2021/06/the-law-of-conservation-of-mass.png

Law Of Conservation Of Mass
https://sciencenotes.org/wp-content/uploads/2023/12/Law-of-Conservation-of-Mass.png
Law Of Conservation Of Mass - Answer and Explanation 1 The law of conservation of mass states that mass has to be accounted for in any type of reaction No mass can be lost and no mass can be gained