Isotope With 1 Proton And 2 Neutrons 2 days ago nbsp 0183 32 isotope one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behaviour but with different atomic masses and physical properties Every chemical element has
Sep 13 2019 nbsp 0183 32 Get the definition of an isotope See examples of isotopes and learn the difference between an isotope and a nuclide of an element What are Isotopes Isotopes can be defined as the variants of chemical elements that possess the same number of protons and electrons but a different number of neutrons In other words isotopes are variants of elements that differ in their nucleon The total number of protons and neutrons numbers due to a difference in the total number of neutrons in their respective nuclei
Isotope With 1 Proton And 2 Neutrons

Isotope With 1 Proton And 2 Neutrons
https://webmis.highland.cc.il.us/~jsullivan/principles-of-general-chemistry-v1.0/section_24/bdcb456214c61f63162fbe680db1e491.jpg

Isotope Notation Overview Examples Expii
https://d20khd7ddkh5ls.cloudfront.net/isotope_notation_calculations.jpeg

Isotop Definisi Jenis Dan Kegunaannya Root Of Science
https://rootofscience.com/blog/wp-content/uploads/2021/10/isotop-hidrogen.png
Feb 4 2020 nbsp 0183 32 Isotope Examples Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons Carbon 12 is a stable isotope while carbon 14 is a radioactive isotope radioisotope Uranium 235 and uranium 238 occur naturally in the Earth s crust Both have long half lives Uranium 234 forms as a decay Aug 19 2022 nbsp 0183 32 They are also used in environmental studies nutrition assessments and forensics Naturally occurring stable isotopes such as isotopes of hydrogen are used by measuring their amounts and ratio in samples of water to determine the water s age and origin understand its history and acknowledge its sources This is known as isotope hydrology
Isotope Formation and Radiation Types Isotopes can either form spontaneously naturally through radioactive decay of a nucleus i e emission of energy in the form of alpha particles beta particles neutrons and photons or artificially by bombarding a stable nucleus with charged particles via accelerators or neutrons in a nuclear reactor Jan 2 2013 nbsp 0183 32 What is the half life of an isotope The half life of an isotope is the average time it takes for it to decay to half its initial amount It is a measure used to describe the stability or decay rate of a radioactive isotope When a radioactive isotope decays it emits subatomic particles or radiation to reach a more stable configuration
More picture related to Isotope With 1 Proton And 2 Neutrons

Definition Of Isotopes
https://d1avenlh0i1xmr.cloudfront.net/f39941af-598d-45a6-a9d7-2c3388b79bc9/36.-isotopes-of-hydrogen-teachoo-01.png
Element Proton Neutron Electron Chart
https://lh6.googleusercontent.com/proxy/rs2S-Rj8rlSVdk-V1vnR6XfXL1GxzwBipBXZfeum8ncIVsRoKqzZmpwPngddPtH1o4XHtcOhfhMXOVE71CwdXTDZjVqJmhWxqXLP4vXpWkg-pY9BNskkPmQ35GYRN2mYOihDWk6x9YhTygdPSIKiqYi0DJNUnnyuaO8TVkiuI1M6wHo=s0-d

How To Calculate The Number Of Protons Neutrons And Electrons
https://i.ytimg.com/vi/65dDZulPhtg/maxresdefault.jpg
Jun 9 2023 nbsp 0183 32 An isotope is an atom of an element that has a different number of neutrons than other atoms of that element Examples of isotopes include hydrogen 1 protium carbon 12 C 12 and carbon 14 C 14 ISOTOPE definition 1 a form of an atom that has a different atomic weight from other forms of the same atom but the Learn more
[desc-10] [desc-11]
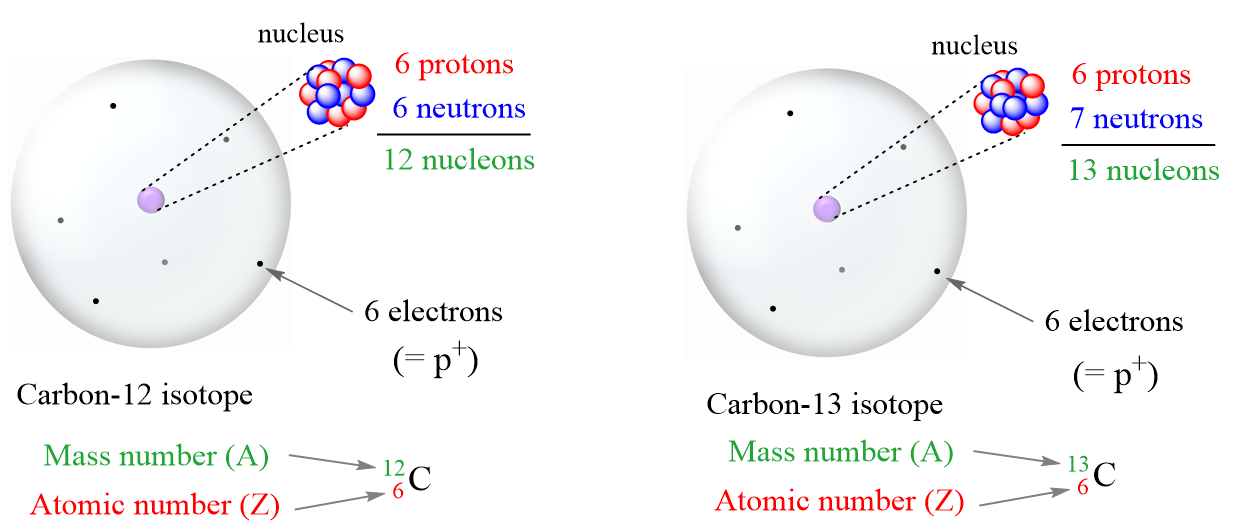
How To Calculate The Number Of Protons Neutrons And Electrons
https://general.chemistrysteps.com/wp-content/uploads/2022/03/Isotopes-protons-neutrons-electrons-and-mass-number.png
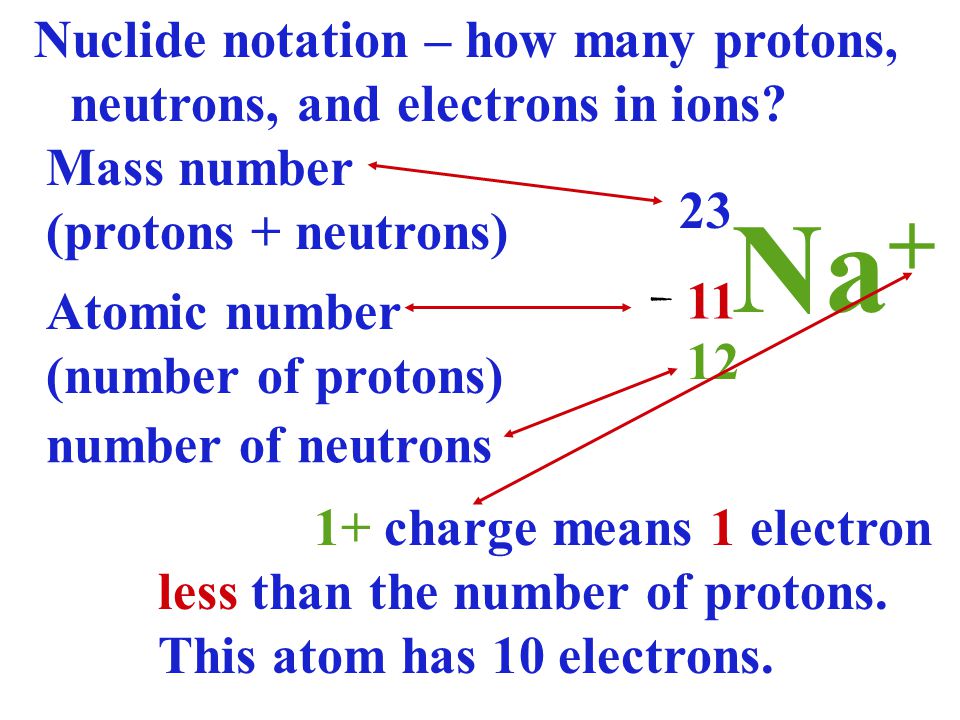
SimplyChemistry C1 1 2 PROTON NUMBER MASS NUMBER IONS ISOTOPES
https://3.bp.blogspot.com/-xjR7quI6CFs/VYLYdrkjBiI/AAAAAAAABeA/I1ufwOUUHhA/s1600/slide_9.jpg
Isotope With 1 Proton And 2 Neutrons - Feb 4 2020 nbsp 0183 32 Isotope Examples Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons Carbon 12 is a stable isotope while carbon 14 is a radioactive isotope radioisotope Uranium 235 and uranium 238 occur naturally in the Earth s crust Both have long half lives Uranium 234 forms as a decay