Ionization Energy Trend Worksheet 3 Answers Ionization in chemistry and physics any process by which electrically neutral atoms or molecules are converted to electrically charged atoms or molecules ions through gaining or losing
Jun 18 2023 nbsp 0183 32 Ionization is a fundamental concept in chemistry and physics that describes the transformation of electrically neutral atoms or molecules into electrically charged ones Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule 2 If an atom or molecule gains an electron it becomes negatively charged an
Ionization Energy Trend Worksheet 3 Answers

Ionization Energy Trend Worksheet 3 Answers
https://i.ytimg.com/vi/My2BQI4Vqqs/maxresdefault.jpg

Dry Lab Periodic Trends Answer Key Name Studocu
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/ad9630f8771021450defeff9fd487460/thumb_1200_1553.png

Periodic Trends In Ionization Energy Chemistry Socratic
https://useruploads.socratic.org/H7ptw5aRSadPRg7SOPwa_ptable orbitals ionization energy.png
Jun 26 2025 nbsp 0183 32 Ionization refers to the process by which a neutral molecule or atom gains or loses electrons resulting in the formation of ions Ionization energy on the other hand is the amount Definition of Ionization What is Ionization Strictly defined ionization is the complete loss of an electron from an atomic or molecular species The resulting species is called an ion In
May 21 2024 nbsp 0183 32 Ionization is when an atom or molecule gains either a positive or negative charge It can occur in one of two ways first when electrons are either gained or lost by a particle May 27 2024 nbsp 0183 32 Ionization is a fundamental concept in electrostatics central to understanding various physical and chemical processes This phenomenon involves the addition or removal
More picture related to Ionization Energy Trend Worksheet 3 Answers

Periodicity Definition In Chemistry
https://sciencenotes.org/wp-content/uploads/2020/07/periodicity-chemistry.jpg

Ionization Energy Definition Chart Periodic Table Trend
https://www.chemistrylearner.com/wp-content/uploads/2021/11/Ionization-Energy-Chart.jpg
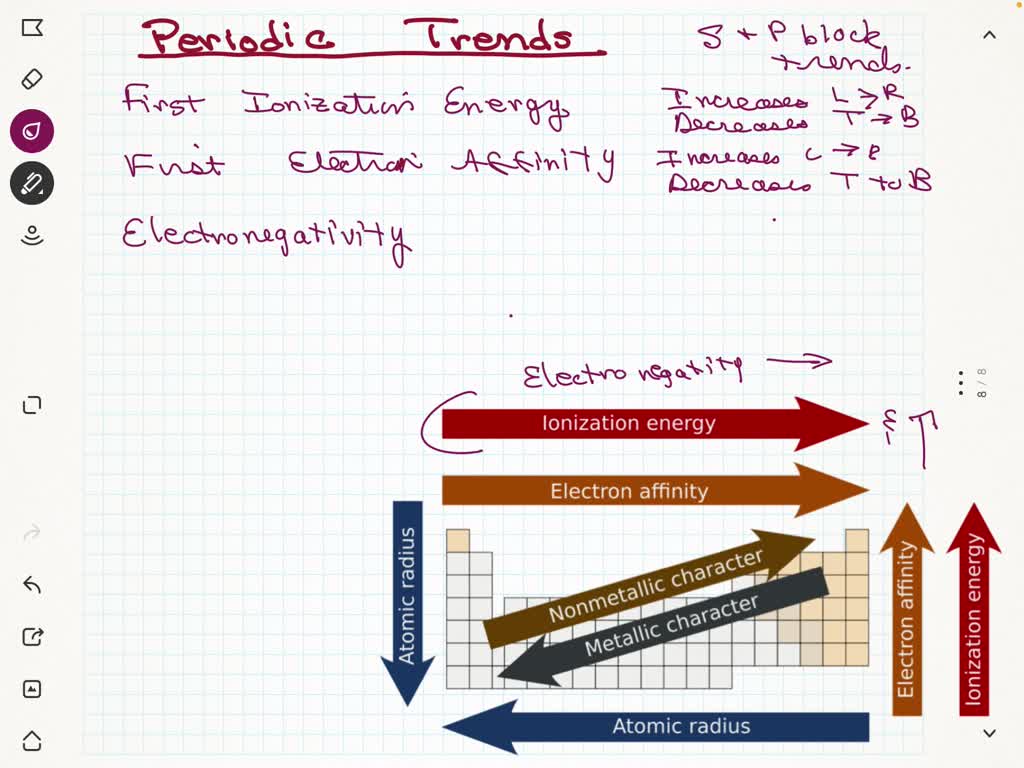
SOLVED Although There Are Notable Exceptions To The Periodic Trends Of
https://cdn.numerade.com/ask_previews/73b216a-2f31-afa8-e7c-4d08ec4248e_large.jpg
Ionization is the process where atoms or molecules gain or lose electrons resulting in charged particles called ions This fundamental concept is essential in various scientific fields including Ionization is any process that changes the electrical balance within an atom If we remove an electron from a stable atom the atom becomes electrically incomplete unbalanced That is
[desc-10] [desc-11]
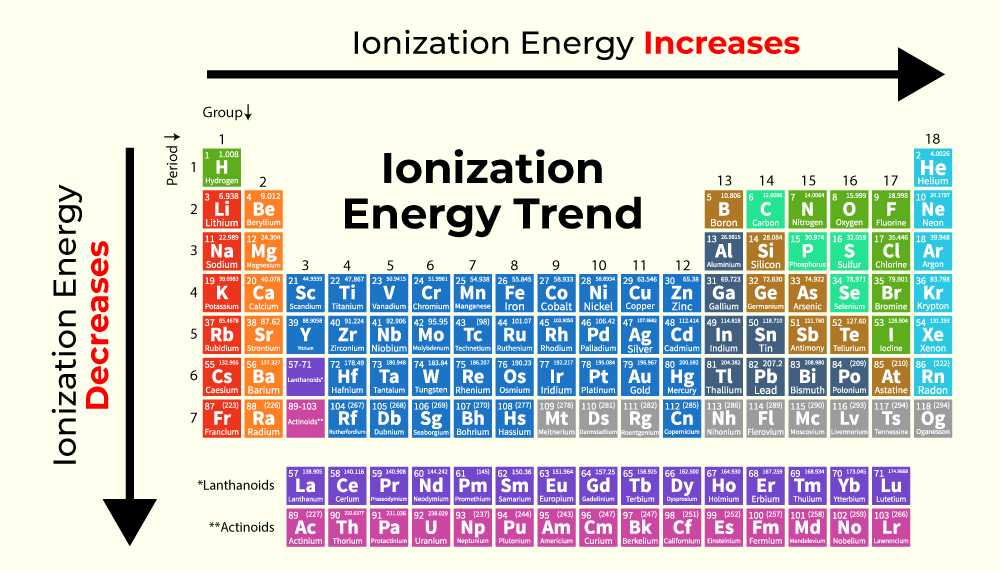
Ionization Energy Definition Formulas And Solved Examples
https://media.geeksforgeeks.org/wp-content/uploads/20230310125901/ionization-energy-trend.png
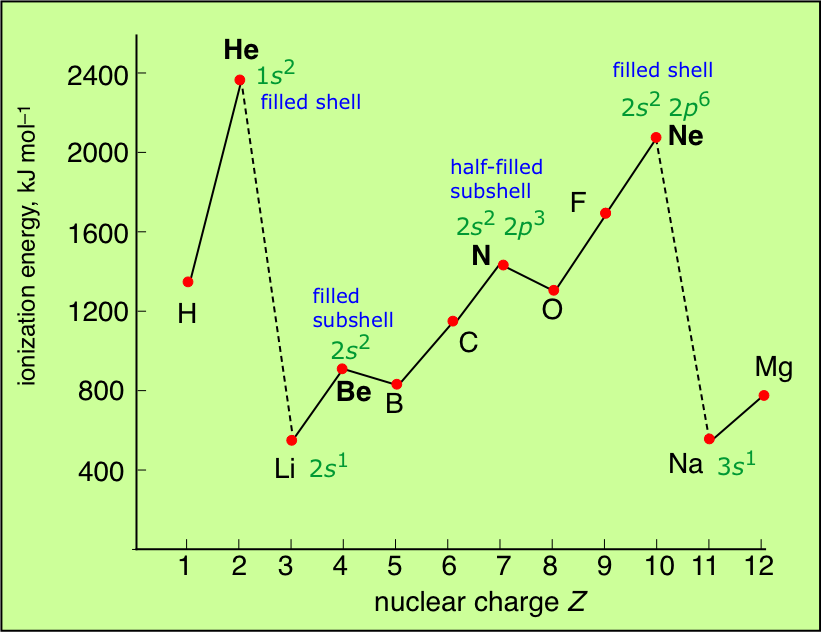
Which Element Has The Highest First Ionization Energy Socratic
https://useruploads.socratic.org/HR96w0abR56Jig3gy4VI_ionization_energies_1-12.png
Ionization Energy Trend Worksheet 3 Answers - May 27 2024 nbsp 0183 32 Ionization is a fundamental concept in electrostatics central to understanding various physical and chemical processes This phenomenon involves the addition or removal