Ionization Energy Trend Explained Ionization in chemistry and physics any process by which electrically neutral atoms or molecules are converted to electrically charged atoms or molecules ions through gaining or losing
Jun 18 2023 nbsp 0183 32 Ionization is a fundamental concept in chemistry and physics that describes the transformation of electrically neutral atoms or molecules into electrically charged ones Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule 2 If an atom or molecule gains an electron it becomes negatively charged an
Ionization Energy Trend Explained

Ionization Energy Trend Explained
https://sciencenotes.org/wp-content/uploads/2020/09/ionization-energies-atoms-768x512.jpg

Second Ionization Energy Periodic Table
https://www.chemistrylearner.com/wp-content/uploads/2021/10/Ionization-Energy-Trend-Periodic-Table.jpg
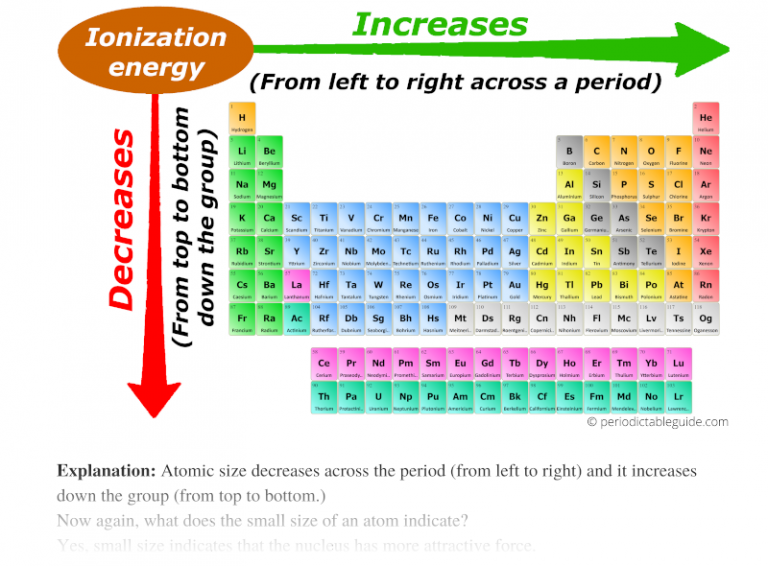
Easy Defiintions For Words Associated With The Periodic Table Bowers
https://periodictableguide.com/wp-content/uploads/2020/12/ionization-energy-trend-in-periodic-table-768x566.png
May 21 2024 nbsp 0183 32 Ionization is when an atom or molecule gains either a positive or negative charge It can occur in one of two ways first when electrons are either gained or lost by a particle May 29 2024 nbsp 0183 32 Ionization is the process by which an atom or a molecule either gains or loses electrons acquiring a net charge and becoming an ion This fundamental concept is pivotal in
Values for the ionization energies of Li L i and Be B e listed in Table 4 4 1 4 4 1 show that successive ionization energies for an element increase steadily that is it takes more energy to Ionization is when an atom or molecule gains or loses electrons turning it into a charged particle called an ion Normally atoms have no charge because they have the same number of positive
More picture related to Ionization Energy Trend Explained

Ionization Energy Trend Science Trends
https://sciencetrends.com/wp-content/uploads/2018/05/b88016d6-ion.png
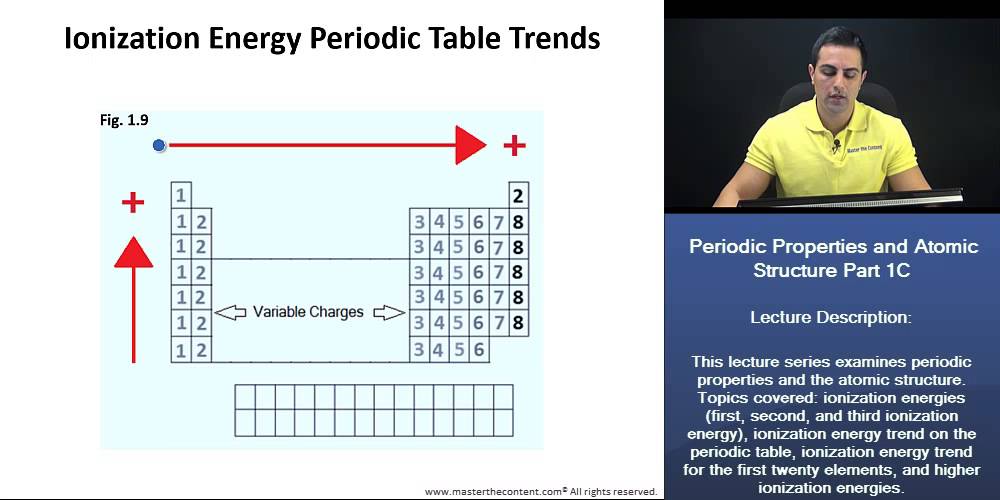
DAT First Second And Third Ionization Energy Ionization Energy
https://i.ytimg.com/vi/em4IlO1-A_8/maxresdefault.jpg

Electron Affinity Trend And Definition Electron Affinity Electrons
https://i.pinimg.com/originals/64/c1/0a/64c10a2710410e3c0fa7f4f83f4eacaf.png
Definition of Ionization What is Ionization Strictly defined ionization is the complete loss of an electron from an atomic or molecular species The resulting species is called an ion In Ionization is a fundamental concept in atomic physics focusing on the process by which an atom or a molecule acquires a positive or negative charge by gaining or losing electrons
[desc-10] [desc-11]
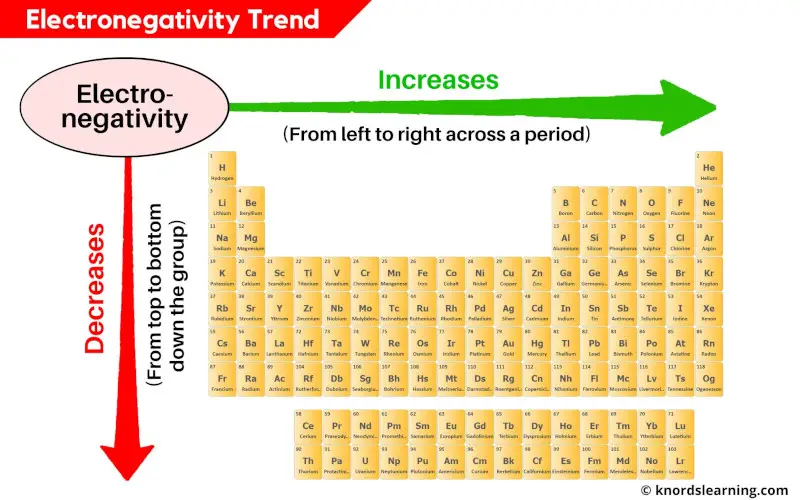
All Periodic Trends Of Periodic Table Simple Explanation
https://knordslearning.com/wp-content/uploads/2022/10/Electronegativity-trend-periodic-table.jpg

Explained 5 Factors Affecting Ionization Energy And Its Trend
https://1.bp.blogspot.com/-nfLqCO7i6SI/Xr-jNfNNmnI/AAAAAAAAARY/c9AQrShBKrkdk6avD-EQFUS30EHnxI11wCPcBGAYYCw/s1600/2-min.jpg
Ionization Energy Trend Explained - Ionization is when an atom or molecule gains or loses electrons turning it into a charged particle called an ion Normally atoms have no charge because they have the same number of positive