Ideal Gas Law Worksheet Review Ideal Gas Law PV nRT which allows scientists to now calculate the number of moles of a gas along with pressure temperature and volume In addition to moles of a gas we can now extend the calculations to include grams or molecules Calculate the molar mass or density of a gas
Ideal Gas Law worksheet with practice problems Calculate moles volume and pressure using PV nRT High School chemistry review The ideal gas law states that PV nRT where P is the pressure of a gas V is the volume of the gas n is the number of moles of gas present R is the ideal gas constant and T is the temperature of the gas in Kelvins
Ideal Gas Law Worksheet Review

Ideal Gas Law Worksheet Review
https://s3.studylib.net/store/data/007410447_1-5cda4427f25c65f8b011c0be8353e592.png
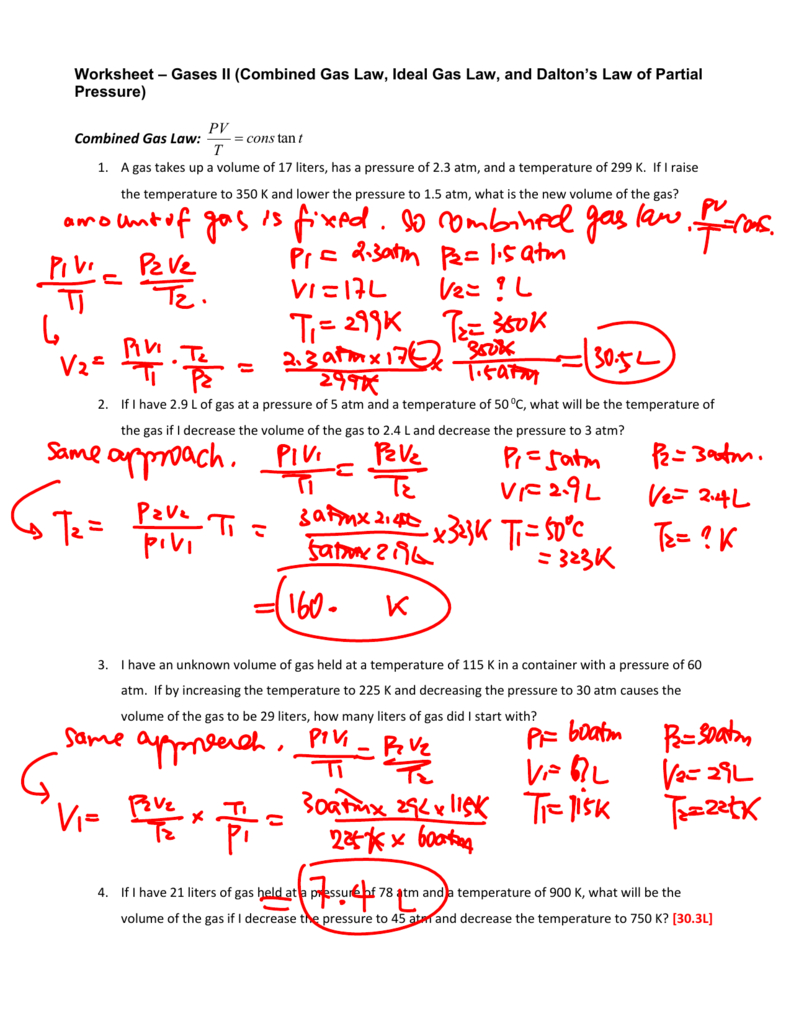
Combined Gas Law Worksheet Answer Key Db excel
https://db-excel.com/wp-content/uploads/2019/09/worksheet-gas-laws-ii-answers-1.png
Gas Laws Worksheet 2 Boyles Charles And Combined Gases Pressure
https://imgv2-1-f.scribdassets.com/img/document/252970357/original/aa3bf77cc0/1587693962?v=1
Using the Ideal Gas Equation in Changing or Constant Environmental Conditions 1 If you were to take a volleyball scuba diving with you what would be its new volume if it started at the surface with a volume of 2 00L under a pressure of 752 0 mmHg and a temperature of 20 0 176 C On your dive you take it to a place where the pressure is 2943 One mole of gas occupies 22 414 L at a pressure of 1 000 atm and a temperature of 0 176 C 273 15 K This is known as standard temperature and pressure or STP Use the ideal gas law to work out the value of the universal gas constant R and its units
Use your knowledge of the ideal and combined gas laws to solve the following problems If it involves moles or grams it must be PV nRT 1 If four moles of a gas at a pressure of 5 4 atmospheres have a volume of 120 liters what is the temperature The ideal gas law states that PV nRT where P is the pressure of a gas V is the volume of the gas n is the number of moles of gas present R is the ideal gas constant and T is the temperature of the gas in Kelvins
More picture related to Ideal Gas Law Worksheet Review

13 Best Images Of Pressure Problems Worksheet Answer Key
http://www.worksheeto.com/postpic/2015/09/ideal-gas-law-worksheet-answers_223443.jpg

Chemistry Lessons Science Notes High School Science
https://i.pinimg.com/originals/b4/c6/0e/b4c60ec55f2c07a49d3b2b4cef88a7b8.jpg

Best Images Of Chemistry Gas Laws Worksheet Ideal Gas Law Worksheet
http://mrphelpsbrhs.weebly.com/uploads/6/4/1/1/6411868/p.24_ideal_gas_law.jpg
Worksheets Involving Gas Laws and Thermodynamics These worksheets are in pdf format or as Microsoft Word files If you don t have MS Word you can download the latest version of the Adobe Acrobat reader for free by clicking HERE Chemistry The Ideal Gas Law Directions Solve each of the following problems Show your work including proper units to earn full credit 1 If 3 7 moles of propane are at a temperature of 28oC and are under 154 2 kPa of pressure what volume does the sample occupy 2
The Ideal Gas Law relates the pressure temperature volume and mass of a gas through the gas constant R The rate of effusion diffusion of two gases A and B are inversely proportional to the square roots of their formula masses Nov 22 2020 nbsp 0183 32 These are Ideal Gas Law problems and these are both Combined Gas Laws and Ideal Gas Law Problems This worksheet is a review of all the gas laws Have students try this Gas Laws Magic Square Do this Gas Laws crossword puzzle or

More Gas Laws Worksheet Charles Law Worksheet Answer Key Chemistry
https://i.pinimg.com/originals/b2/3f/de/b23fde7086ac9160728b068d0a3c3777.gif

Ideal Gas Law Worksheet Answers
https://sp-uploads.s3.amazonaws.com/uploads/services/1881702/20210809112358_6111104e17b8b_ideal_gas_law_worksheet_2_answer_page1.png
Ideal Gas Law Worksheet Review - Use your knowledge of the ideal and combined gas laws to solve the following problems If it involves moles or grams it must be PV nRT 1 If four moles of a gas at a pressure of 5 4 atmospheres have a volume of 120 liters what is the temperature
