Ideal Gas Law Practice Problems May 28 2020 nbsp 0183 32 What is the density of laughing gas dinitrogen monoxide N 2 O at a temperature of 325 K and a pressure of 113 0 kPa Calculate the density of Freon 12 CF 2 Cl 2 at 30 0 176 C and 0 954 atm Which is denser at the same temperature and pressure dry air or air saturated with water vapor Explain
2 What is the volume of 1 00 mole of a gas at standard temperature and pressure 3 A 113L sample of helium at 27 176 C is cooled at constant pressure to 78 0 176 C Calculate the new volume of the helium 4 What volume of He is occupied by 2 35 mol of This quiz helps you practice gas law calculations using Boyle s Law Charles Law Gay Lussac s Law and the Ideal Gas Law PV nRT
Ideal Gas Law Practice Problems

Ideal Gas Law Practice Problems
https://s3.studylib.net/store/data/008423281_1-c32115dedb373c7f8ffe4fb72e5478df.png

Ideal Gas Law Problems And Answers
https://i0.wp.com/dj1hlxw0wr920.cloudfront.net/userfiles/wyzfiles/7a1e2bbb-35a4-415f-afb8-81721255c1da.png

The Combined Gas Law Slidesharetrick
https://i.ytimg.com/vi/CgrCFRsWMtI/maxresdefault.jpg
May 28 2020 nbsp 0183 32 Sometimes leaving a bicycle in the sun on a hot day will cause a blowout Why As temperature of a gas increases pressure will also increase based on the ideal gas law The volume of the tire can only expand so much before the The ideal gas laws show the correlation of the temperature pressure volume and the amount of a gas Although this is not how the laws were determined I found my students grasping the concepts a lot easier using the following model
Ideal Gas Law and Stoichiometry Name Use the following reaction to answer the next few questions 2 C8H18 l 25 O2 g gt 16 CO2 g 18 H2O g The above reaction is the reaction between gasoline octane and oxygen that occurs inside automobile engines IB Chemistry II Practice Problems Ideal Gas Law 1 Which change in conditions would increase the volume of a fixed mass of gas 2 All of the following are characteristic properties of gases EXCEPT A They can expand without limit B They diffuse readily C They are easily compressed D They have high densities 3
More picture related to Ideal Gas Law Practice Problems

DOC ANSWER KEY For More Gas Law Practice Problems Ideal Gas Law
https://0.academia-photos.com/attachment_thumbnails/53187908/mini_magick20180816-15329-jymnyv.png?1534405965

Ideal Gas Law Problems
https://cdn.slidesharecdn.com/ss_thumbnails/idealgaslawproblems-100405160841-phpapp01-thumbnail-4.jpg?cb=1270483733
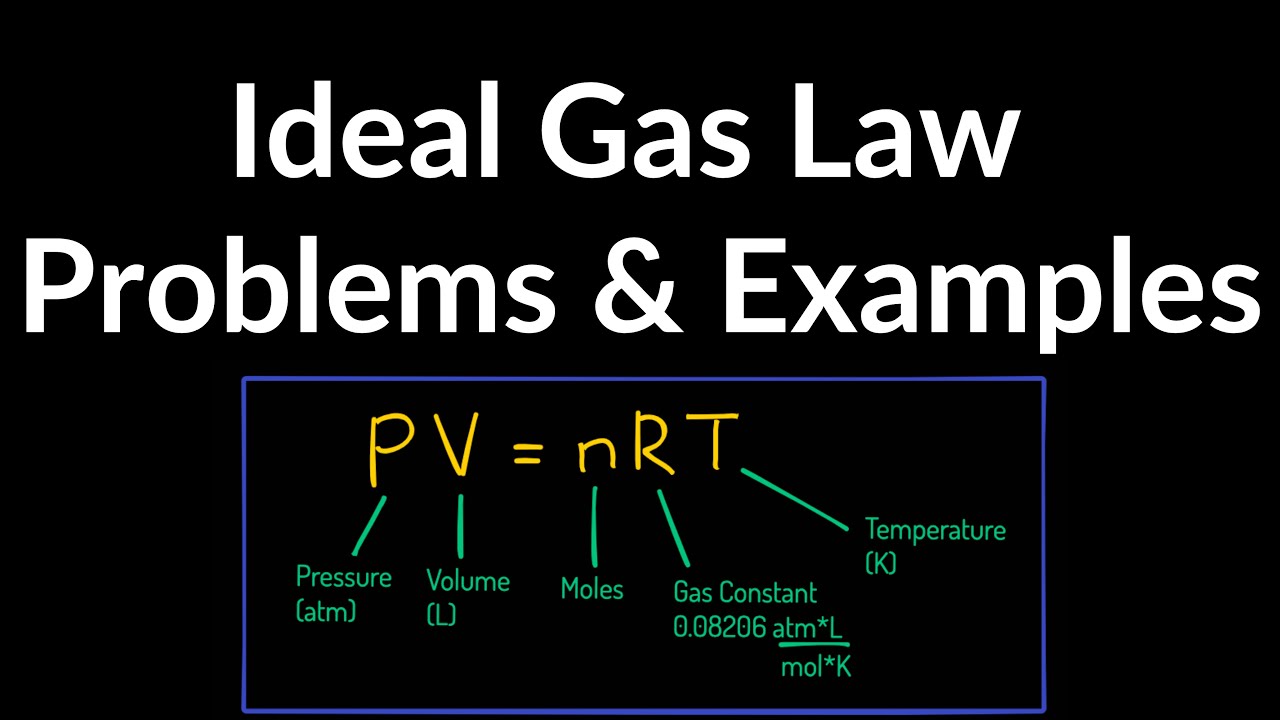
Ideal Gas Law Practice Problems Examples YouTube
https://i.ytimg.com/vi/tqp9eMkynB8/maxresdefault.jpg
The document provides practice problems for applying the ideal gas law which relates pressure volume temperature and amount of gas in an enclosed system Students are instructed to complete the practice problems on looseleaf paper showing all Apr 23 2019 nbsp 0183 32 Decide which gas law should be used to solve each of the following a Calculate the final volume of a sample of gas that has an initial volume of 7 10 L at STP if the temperature and pressure are changed to 33 o C and 696 torr b Calculate the volume of 0 977 mol of gas at 33 o C and 792 torr Solution a The combined gas law can be used
[desc-10] [desc-11]
Determining Gas Properties Through Gas Law Calculations PDF Gases
https://imgv2-2-f.scribdassets.com/img/document/247039702/original/19a05f6b4c/1705703005?v=1

Worked Chemistry Problems Ideal Gas Law Thoughtco
http://i1.ytimg.com/vi/C6zluQSNc2c/maxresdefault.jpg
Ideal Gas Law Practice Problems - IB Chemistry II Practice Problems Ideal Gas Law 1 Which change in conditions would increase the volume of a fixed mass of gas 2 All of the following are characteristic properties of gases EXCEPT A They can expand without limit B They diffuse readily C They are easily compressed D They have high densities 3
